piezo2b regulates vertebrate light touch response
- PMID: 24155313
- PMCID: PMC6618434
- DOI: 10.1523/JNEUROSCI.0522-13.2013
piezo2b regulates vertebrate light touch response
Abstract
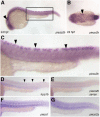
The sense of touch allows an organism to detect and respond to physical environmental stimuli. Mechanosensitive proteins play a crucial role in this process by converting the mechanical cue into a biological response. Recently, the Piezo family of stretch-activated ion channels has been identified as genuine mechanosensitive proteins. We set out to determine whether any of these genes are involved in touch response during zebrafish development. In situ hybridization indicates that piezo2b is specifically expressed in a subset of neurons (Rohon-Beard cells) responsible for detecting light touch. Using morpholino-mediated knockdown, we specifically targeted piezo2b and determined that it is involved in mediating touch-evoked response.
Keywords: Piezo2.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
