Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons
- PMID: 24155314
- PMCID: PMC3807031
- DOI: 10.1523/JNEUROSCI.0890-13.2013
Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons
Abstract
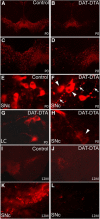
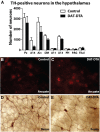
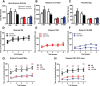
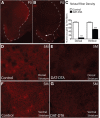
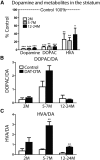
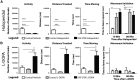
The loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and consequent depletion of striatal dopamine are known to underlie the motor deficits observed in Parkinson's disease (PD). Adaptive changes in dopaminergic terminals and in postsynaptic striatal neurons can compensate for significant losses of striatal dopamine, resulting in preservation of motor behavior. In addition, compensatory changes independent of striatal dopamine have been proposed based on PD therapies that modulate nondopaminergic circuits within the basal ganglia. We used a genetic strategy to selectively destroy dopaminergic neurons in mice during development to determine the necessity of these neurons for the maintenance of normal motor behavior in adult and aged mice. We find that loss of 90% of SNc dopaminergic neurons and consequent depletion of >95% of striatal dopamine does not result in changes in motor behavior in young-adult or aged mice as evaluated by an extensive array of motor behavior tests. Treatment of aged mutant mice with the dopamine receptor antagonist haloperidol precipitated motor behavior deficits in aged mutant mice, indicating that <5% of striatal dopamine is sufficient to maintain motor function in these mice. We also found that mutant mice exhibit an exaggerated response to l-DOPA compared with control mice, suggesting that preservation of motor function involves sensitization of striatal dopamine receptors. Our results indicate that congenital loss of dopaminergic neurons induces remarkable adaptions in the nigrostriatal system where limited amounts of dopamine in the dorsal striatum can maintain normal motor function.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- DK081644/DK/NIDDK NIH HHS/United States
- R01 DK082531/DK/NIDDK NIH HHS/United States
- R01 NS048602/NS/NINDS NIH HHS/United States
- DK082531/DK/NIDDK NIH HHS/United States
- P30-DK079333/DK/NIDDK NIH HHS/United States
- AG013370/AG/NIA NIH HHS/United States
- R01 DK081644/DK/NIDDK NIH HHS/United States
- R21 NS059566/NS/NINDS NIH HHS/United States
- P30 DK079333/DK/NIDDK NIH HHS/United States
- NS048602/NS/NINDS NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- R56 NS048602/NS/NINDS NIH HHS/United States
- R21NS059566/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
