Actin network disassembly powers dissemination of Listeria monocytogenes
- PMID: 24155331
- PMCID: PMC3874788
- DOI: 10.1242/jcs.140038
Actin network disassembly powers dissemination of Listeria monocytogenes
Abstract
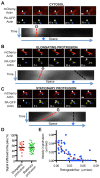
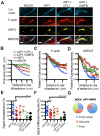
Several bacterial pathogens hijack the actin assembly machinery and display intracellular motility in the cytosol of infected cells. At the cell cortex, intracellular motility leads to bacterial dissemination through formation of plasma membrane protrusions that resolve into vacuoles in adjacent cells. Here, we uncover a crucial role for actin network disassembly in dissemination of Listeria monocytogenes. We found that defects in the disassembly machinery decreased the rate of actin tail turnover but did not affect the velocity of the bacteria in the cytosol. By contrast, defects in the disassembly machinery had a dramatic impact on bacterial dissemination. Our results suggest a model of L. monocytogenes dissemination in which the disassembly machinery, through local recycling of the actin network in protrusions, fuels continuous actin assembly at the bacterial pole and concurrently exhausts cytoskeleton components from the network distal to the bacterium, which enables membrane apposition and resolution of protrusions into vacuoles.
Keywords: AIP1; ARP2/3; Actin assembly; Actin network disassembly; CFL1; GMFB; Listeria; WDR1.
Figures





References
-
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. (1992). A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 11, 1981–1990 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

