CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection
- PMID: 24155379
- PMCID: PMC3911719
- DOI: 10.1128/JVI.02077-13
CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection
Abstract
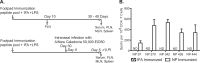
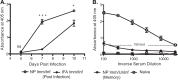
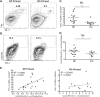
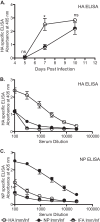
Influenza virus vaccination strategies are focused upon the elicitation of protective antibody responses through administration of viral protein through either inactivated virions or live attenuated virus. Often overlooked in this strategy is the CD4 T cell response: how it develops into memory, and how it may support future primary B cell responses to heterologous infection. Through the utilization of a peptide-priming regimen, this study describes a strategy for developing CD4 T cell memory with the capacity to robustly expand in the lung-draining lymph node after live influenza virus infection. Not only were frequencies of antigen-specific CD4 T cells enhanced, but these cells also supported an accelerated primary B cell response to influenza virus-derived protein, evidenced by high anti-nucleoprotein (NP) serum antibody titers early, while there is still active viral replication ongoing in the lung. NP-specific antibody-secreting cells and heightened frequencies of germinal center B cells and follicular T helper cells were also readily detectable in the draining lymph node. Surprisingly, a boosted memory CD4 T cell response was not sufficient to provide intermolecular help for antibody responses. Our study demonstrates that CD4 T cell help is selective and limiting to the primary antibody response to influenza virus infection and that preemptive priming of CD4 T cell help can promote effective and rapid conversion of naive B cells to mature antibody-secreting cells.
Figures


References
-
- Jackson LA, Gaglani MJ, Keyserling HL, Balser J, Bouveret N, Fries L, Treanor JJ. 2010. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect. Dis. 10:71. 10.1186/1471-2334-10-71 - DOI - PMC - PubMed
-
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, Petrie JG, Lofthus G, Meece JK, Williams JV, Berman L, Breese Hall C, Monto AS, Griffin MR, Belongia E, Shay DK. 2012. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin. Infect. Dis. 55:951–959. 10.1093/cid/cis574 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

