A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2
- PMID: 24155388
- PMCID: PMC3911713
- DOI: 10.1128/JVI.01141-13
A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2
Abstract
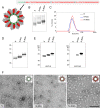
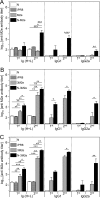
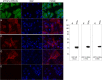
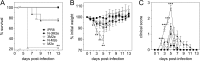
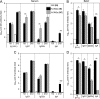
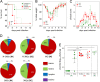
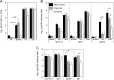
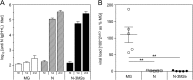
In this study, subnucleocapsid nanorings formed by the recombinant nucleoprotein (N) of the respiratory syncytial virus were evaluated as a platform to anchor heterologous antigens. The ectodomain of the influenza virus A matrix protein 2 (M2e) is highly conserved and elicits protective antibodies when it is linked to an immunogenic carrier, making it a promising target to develop universal influenza vaccines. In this context, one or three M2e copies were genetically linked to the C terminus of N to produce N-M2e and N-3M2e chimeric recombinant nanorings. Mice were immunized intranasally with N-M2e or N-3M2e or with M2e or 3M2e control peptides. N-3M2e-vaccinated mice showed the strongest mucosal and systemic antibody responses. These mice presented a reduced viral load and minor weight loss, and all survived upon challenge with influenza virus A/PR8/34 (H1N1) (PR8). We compared the intranasal route to the subcutaneous route of N-3M2e immunization. Only the intranasal route induced a strong local IgA response and led to the protection of mice upon challenge. Finally, we demonstrated that the induction of anti-M2e antibodies by N-3M2e is not impaired by preexisting anti-N immunity. Overall, these results show that the N nanoring is a potent carrier for mucosal delivery of vaccinal antigens.
Figures
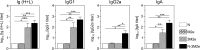


Similar articles
-
Immunogenicity and Protective Potential of Mucosal Vaccine Formulations Based on Conserved Epitopes of Influenza A Viruses Fused to an Innovative Ring Nanoplatform in Mice and Chickens.Front Immunol. 2021 Nov 11;12:772550. doi: 10.3389/fimmu.2021.772550. eCollection 2021. Front Immunol. 2021. PMID: 34868036 Free PMC article.
-
Combination of conserved recombinant proteins (NP & 3M2e) formulated with Alum protected BALB/c mice against influenza A/PR8/H1N1 virus challenge.Biotechnol Lett. 2021 Nov;43(11):2137-2147. doi: 10.1007/s10529-021-03174-2. Epub 2021 Sep 7. Biotechnol Lett. 2021. PMID: 34491470
-
Recombinant baculovirus vaccine containing multiple M2e and adjuvant LTB induces T cell dependent, cross-clade protection against H5N1 influenza virus in mice.Vaccine. 2016 Jan 27;34(5):622-629. doi: 10.1016/j.vaccine.2015.12.039. Epub 2015 Dec 23. Vaccine. 2016. PMID: 26724200
-
Intranasal Nanovaccine Confers Homo- and Hetero-Subtypic Influenza Protection.Small. 2018 Mar;14(13):e1703207. doi: 10.1002/smll.201703207. Epub 2018 Feb 12. Small. 2018. PMID: 29430819
-
Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse.Virol J. 2007 Oct 31;4:118. doi: 10.1186/1743-422X-4-118. Virol J. 2007. PMID: 17974006 Free PMC article.
Cited by
-
Combination of M2e peptide with stalk HA epitopes of influenza A virus enhances protective properties of recombinant vaccine.PLoS One. 2018 Aug 23;13(8):e0201429. doi: 10.1371/journal.pone.0201429. eCollection 2018. PLoS One. 2018. PMID: 30138320 Free PMC article.
-
Immunogenicity and Protective Potential of Mucosal Vaccine Formulations Based on Conserved Epitopes of Influenza A Viruses Fused to an Innovative Ring Nanoplatform in Mice and Chickens.Front Immunol. 2021 Nov 11;12:772550. doi: 10.3389/fimmu.2021.772550. eCollection 2021. Front Immunol. 2021. PMID: 34868036 Free PMC article.
-
Emerging Advances of Nanotechnology in Drug and Vaccine Delivery against Viral Associated Respiratory Infectious Diseases (VARID).Int J Mol Sci. 2021 Jun 28;22(13):6937. doi: 10.3390/ijms22136937. Int J Mol Sci. 2021. PMID: 34203268 Free PMC article. Review.
-
Nanotechnology against the novel coronavirus (severe acute respiratory syndrome coronavirus 2): diagnosis, treatment, therapy and future perspectives.Nanomedicine (Lond). 2021 Mar;16(6):497-516. doi: 10.2217/nnm-2020-0441. Epub 2021 Mar 8. Nanomedicine (Lond). 2021. PMID: 33683164 Free PMC article. Review.
-
M2e-Based Universal Influenza A Vaccines.Vaccines (Basel). 2015 Feb 13;3(1):105-36. doi: 10.3390/vaccines3010105. Vaccines (Basel). 2015. PMID: 26344949 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

