Discovery of novel ribonucleoside analogs with activity against human immunodeficiency virus type 1
- PMID: 24155391
- PMCID: PMC3911745
- DOI: 10.1128/JVI.02444-13
Discovery of novel ribonucleoside analogs with activity against human immunodeficiency virus type 1
Abstract
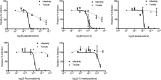
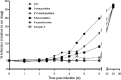
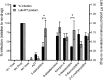
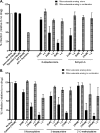
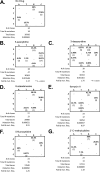
Reverse transcription is an important early step in retrovirus replication and is a key point targeted by evolutionarily conserved host restriction factors (e.g., APOBEC3G, SamHD1). Human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) is a major target of antiretroviral drugs, and concerns regarding drug resistance and off-target effects have led to continued efforts for identifying novel approaches to targeting HIV-1 RT. Several observations, including those obtained from monocyte-derived macrophages, have argued that ribonucleotides and their analogs can, intriguingly, impact reverse transcription. For example, we have previously demonstrated that 5-azacytidine has its greatest antiviral potency during reverse transcription by enhancement of G-to-C transversion mutations. In the study described here, we investigated a panel of ribonucleoside analogs for their ability to affect HIV-1 replication during the reverse transcription process. We discovered five ribonucleosides-8-azaadenosine, formycin A, 3-deazauridine, 5-fluorocytidine, and 2'-C-methylcytidine-that possess anti-HIV-1 activity, and one of these (i.e., 3-deazauridine) has a primary antiviral mechanism that involves increased HIV-1 mutational loads, while quantitative PCR analysis determined that the others resulted in premature chain termination. Taken together, our findings provide the first demonstration of a series of ribonucleoside analogs that can target HIV-1 reverse transcription with primary antiretroviral mechanisms that include premature termination of viral DNA synthesis or enhanced viral mutagenesis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

