Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells
- PMID: 24155397
- PMCID: PMC3911690
- DOI: 10.1128/JVI.01788-13
Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells
Abstract
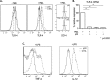
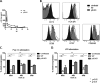
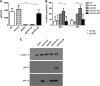
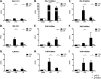
The human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p30 protein, essential for virus infectivity in vivo, is required for efficient infection of human dendritic cells (DCs) but not B and T cells in vitro. We used a human monocytic cell line, THP-1, and dendritic cells to study the mechanism of p30 and p12/p8 requirements in these cell types. p30 inhibited the expression of interferon (IFN)-responsive genes (ISG) following stimulation by lipopolysaccharide (LPS) of Toll-like receptor 4 (TLR4) and by poly(I·C) of TLR3 but not of TLR7/8 with imiquimod. Results with THP-1 mirrored those for ex vivo human primary monocytes and monocyte-derived dendritic cells (Mo-mDC). The effect of p30 on TLR signaling was also demonstrated by ablating its expression within a molecular clone of HTLV-1. HTLV-1 infection of monocytes inhibited TLR3- and TLR4-induced ISG expression by 50 to 90% depending on the genes, whereas the isogenic clone p30 knockout virus was less effective at inhibiting TLR3 and TRL4 signaling and displayed lower infectivity. Viral expression and inhibition of ISG transcription was, however, rescued by restoration of p30 expression. A chromatin immunoprecipitation assay demonstrated that p30 inhibits initiation and elongation of PU.1-dependent transcription of IFN-α1, IFN-β, and TLR4 genes upon TLR stimulation. In contrast, experiments conducted with p12/p8 did not demonstrate an effect on ISG expression. These results provide a mechanistic explanation of the requirement of p30 for HTLV-1 infectivity in vivo, suggest that dampening interferon responses in monocytes and DCs is specific for p30, and represent an essential early step for permissive HTLV-1 infection and persistence.
Figures


References
-
- Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. 1990. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune. Defic. Syndr. 3:1096–1101 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

