Silencing of atp6v1c1 prevents breast cancer growth and bone metastasis
- PMID: 24155661
- PMCID: PMC3805834
- DOI: 10.7150/ijbs.6030
Silencing of atp6v1c1 prevents breast cancer growth and bone metastasis
Abstract
Previous studies have shown that Atp6v1c1, a regulator of the assembly of the V0 and V1 domains of the V-ATPase complex, is up-regulated in metastatic oral tumors. Despite these studies, the function of Atp6v1c1 in tumor growth and metastasis is still unknown. Atp6v1c1's expression in metastatic oral squamous cell carcinoma indicates that Atp6v1c1 has an important function in cancer growth and metastasis. We hypothesized that elevated expression of Atp6v1c1 is essential to cancer growth and metastasis and that Atp6v1c1 promotes cancer growth and metastasis through activation of V-ATPase activity. To test this hypothesis, a Lentivirus-mediated RNAi knockdown approach was used to study the function of Atp6v1c1 in mouse 4T1 mammary tumor cell proliferation and migration in vitro and cancer growth and metastasis in vivo. Our data revealed that silencing of Atp6v1c1 in 4T1 cancer cells inhibited lysosomal acidification and severely impaired 4T1 cell growth, migration, and invasion through Matrigel in vitro. We also show that Atp6v1c1 knockdown with Lenti-c1s3, a lentivirus targeting Atp6v1c1 for shRNA mediated knockdown, can significantly inhibit 4T1 xenograft tumor growth, metastasis, and osteolytic lesions in vivo. Our study demonstrates that Atp6v1c1 may promote breast cancer growth and bone metastasis through regulation of lysosomal V-ATPase activity, indicating that Atp6v1c1 may be a viable target for breast cancer therapy and silencing of Atp6v1c1 may be an innovative therapeutic approach for the treatment and prevention of breast cancer growth and metastasis.
Keywords: Atp6v1c1 (C1); V-ATPase; breast cancer; lysosomal acidification; tumor bone metastasis..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
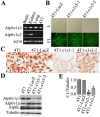

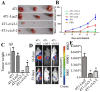
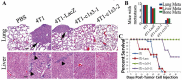
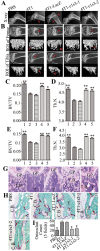
References
-
- Martinez-Zaguilan R, Martinez GM, Gomez A, Hendrix MJ, Gillies RJ. Distinct regulation of pHin and [Ca2+]in in human melanoma cells with different metastatic potential. J.Cell Physiol. 1998 Jul;176(1):196–205. - PubMed
-
- Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am.J.Physiol. 1993 Oct;265(4 Pt 1):C1015–C1029. - PubMed
-
- Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am.J.Physiol Cell Physiol. 2004 Jun;286(6):C1443–C1452. - PubMed
-
- Martinez-Zaguilan R, Raghunand N, Lynch RM, Bellamy W, Martinez GM, Rojas B, Smith D, Dalton WS, Gillies RJ. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem.Pharmacol. 1999 May 1;57(9):1037–46. - PubMed
-
- De MA, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L. et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007 Jun 1;67(11):5408–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

