Toward a self-wired active reconstruction of the hippocampal trisynaptic loop: DG-CA3
- PMID: 24155693
- PMCID: PMC3800815
- DOI: 10.3389/fncir.2013.00165
Toward a self-wired active reconstruction of the hippocampal trisynaptic loop: DG-CA3
Abstract
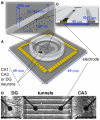
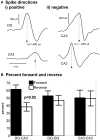
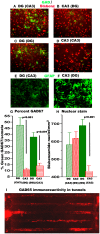
The mammalian hippocampus functions to encode and retrieve memories by transiently changing synaptic strengths, yet encoding in individual subregions for transmission between regions remains poorly understood. Toward the goal of better understanding the coding in the trisynaptic pathway from the dentate gyrus (DG) to the CA3 and CA1, we report a novel microfabricated device that divides a micro-electrode array into two compartments of separate hippocampal network subregions connected by axons that grow through 3 × 10 × 400 μm tunnels. Gene expression by qPCR demonstrated selective enrichment of separate DG, CA3, and CA1 subregions. Reconnection of DG to CA3 altered burst dynamics associated with marked enrichment of GAD67 in DG and GFAP in CA3. Surprisingly, DG axon spike propagation was preferentially unidirectional to the CA3 region at 0.5 m/s with little reverse transmission. Therefore, select hippocampal subregions intrinsically self-wire in anatomically appropriate patterns and maintain their distinct subregion phenotype without external inputs.
Keywords: GAD67; GFAP; burst; dentate gyrus; multielectrode array.
Figures


References
-
- Amaral D. G., Lavenex P. (2006). Hippocampal neuroanatomy, in The Hippocampus Book, eds Andersen P., Morris R., Amaral D., Bliss T., O'Keefe J. (New York, NY: Oxford University Press; ), 37–114
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

