Optogenetic stimulation effectively enhances intrinsically generated network synchrony
- PMID: 24155695
- PMCID: PMC3805139
- DOI: 10.3389/fncir.2013.00167
Optogenetic stimulation effectively enhances intrinsically generated network synchrony
Abstract
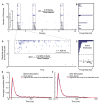
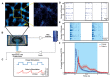
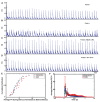
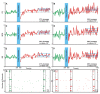
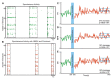
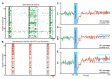
Synchronized bursting is found in many brain areas and has also been implicated in the pathophysiology of neuropsychiatric disorders such as epilepsy, Parkinson's disease, and schizophrenia. Despite extensive studies of network burst synchronization, it is insufficiently understood how this type of network wide synchronization can be strengthened, reduced, or even abolished. We combined electrical recording using multi-electrode array with optical stimulation of cultured channelrhodopsin-2 transducted hippocampal neurons to study and manipulate network burst synchronization. We found low frequency photo-stimulation protocols that are sufficient to induce potentiation of network bursting, modifying bursting dynamics, and increasing interneuronal synchronization. Surprisingly, slowly fading-in light stimulation, which substantially delayed and reduced light-driven spiking, was at least as effective in reorganizing network dynamics as much stronger pulsed light stimulation. Our study shows that mild stimulation protocols that do not enforce particular activity patterns onto the network can be highly effective inducers of network-level plasticity.
Keywords: bursting; multi-electrode arrays; network-level plasticity; optogenetics; synchronization.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

