3-D neurohistology of transparent tongue in health and injury with optical clearing
- PMID: 24155698
- PMCID: PMC3805177
- DOI: 10.3389/fnana.2013.00036
3-D neurohistology of transparent tongue in health and injury with optical clearing
Abstract
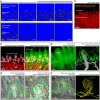
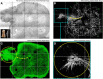
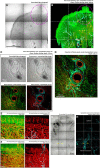
Tongue receives extensive innervation to perform taste, sensory, and motor functions. Details of the tongue neuroanatomy and its plasticity in response to injury offer insights to investigate tongue neurophysiology and pathophysiology. However, due to the dispersed nature of the neural network, standard histology cannot provide a global view of the innervation. We prepared transparent mouse tongue by optical clearing to reveal the spatial features of the tongue innervation and its remodeling in injury. Immunostaining of neuronal markers, including PGP9.5 (pan-neuronal marker), calcitonin gene-related peptide (sensory nerves), tyrosine hydroxylase (sympathetic nerves), and vesicular acetylcholine transporter (cholinergic parasympathetic nerves and neuromuscular junctions), was combined with vessel painting and nuclear staining to label the tissue network and architecture. The tongue specimens were immersed in the optical-clearing solution to facilitate photon penetration for 3-dimensiontal (3-D) confocal microscopy. Taking advantage of the transparent tissue, we simultaneously revealed the tongue microstructure and innervation with subcellular-level resolution. 3-D projection of the papillary neurovascular complex and taste bud innervation was used to demonstrate the spatial features of tongue mucosa and the panoramic imaging approach. In the tongue injury induced by 4-nitroquinoline 1-oxide administration in the drinking water, we observed neural tissue remodeling in response to the changes of mucosal and muscular structures. Neural networks and the neuromuscular junctions were both found rearranged at the peri-lesional region, suggesting the nerve-lesion interactions in response to injury. Overall, this new tongue histological approach provides a useful tool for 3-D imaging of neural tissues to better characterize their roles with the mucosal and muscular components in health and disease.
Keywords: neural network; neurohistology; neuromuscular junction; optical clearing; papilla; skeletal muscle; tongue innervation; tongue lesion.
Figures


References
-
- Arvidsson U., Riedl M., Elde R., Meister B. (1997). Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 378, 454–467 10.1002/(SICI)1096-9861(19970224)378:4<454::AID-CNE2>3.0.CO;2-1 - DOI - PubMed
-
- Carl Zeiss Microimaging GmbH. (2009). Visualizing the Architecture of Cells and Tissues. Brochures for Laser Scanning Microscopy. Available online at: http://microscopy.zeiss.com/microscopy/en_gb/downloads/brochure-download... [Accessed 06 August 2013].
LinkOut - more resources
Full Text Sources
Other Literature Sources

