Aspirin Blocks EGF-stimulated Cell Viability in a COX-1 Dependent Manner in Ovarian Cancer Cells
- PMID: 24155779
- PMCID: PMC3805995
- DOI: 10.7150/jca.7118
Aspirin Blocks EGF-stimulated Cell Viability in a COX-1 Dependent Manner in Ovarian Cancer Cells
Abstract
Objective: Although aspirin has been associated with a reduction of the risk of cancer when used as a nonsteroidal anti-inflammatory drug, its use to reduce the risk of ovarian cancer is controversial. Ovarian cancer cells usually express high levels of cyclooxygenase-1 (COX)-1. Because aspirin is a rather selective inhibitor of COX-1, the ability of aspirin to reduce the risk of ovarian cancer may be dependent on the level of COX-1 expression in those cells. Furthermore, epidermal growth factor receptor (EGFR) is frequently overexpressed in the malignant phenotype of ovarian cancer leading to increased cell proliferation and survival. Here we investigated if aspirin attenuates EGFR-activated ovarian cancer cell growth in a COX-1 dependent manner.
Methods: Cell viability assays and Western blot analyses were used to determine the effect of aspirin on EGF-stimulated cell proliferation. Gene silencing and gene expression techniques were employed to knockdown or to express COX-1, respectively.
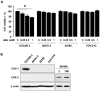
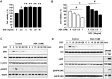
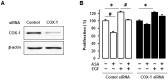
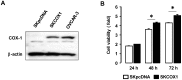
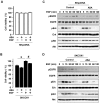
Results: Aspirin inhibited cell viability induced by EGF in a dose dependent manner in COX-1 positive ovarian cancer cells. On the other hand, aspirin had no effect on cell viability in COX-1 negative ovarian cancer cells. In particular, aspirin decreased phosphorylated Akt and Erk activated by EGF. COX-1 silencing in COX-1 positive cells attenuated the inhibitory effect of aspirin on EGF-stimulated cell viability. Furthermore, we developed a COX-1 expressing cell line (SKCOX-1) by stably transfecting COX-1 expression vector into COX-1 negative SKOV-3 cells. SKCOX-1 cells were more responsive to aspirin when compared to cells transfected with empty vector, and decreased EGF-activated Akt and Erk as well as cell viability.
Conclusions: Taken together, aspirin inhibits viability of ovarian cancer cells by blocking phosphorylation of Akt and Erk activated by EGF. Thus it may potentiate the therapeutic efficacy of drugs used to treat COX-1 positive ovarian cancer subsets.
Keywords: Akt; COX-1; EGF; Erk; aspirin; cell viability.; ovarian cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Preferential effect of akt2-dependent signaling on the cellular viability of ovarian cancer cells in response to EGF.J Cancer. 2014 Sep 5;5(8):670-8. doi: 10.7150/jca.9688. eCollection 2014. J Cancer. 2014. PMID: 25258648 Free PMC article.
-
EBP50 inhibits EGF-induced breast cancer cell proliferation by blocking EGFR phosphorylation.Amino Acids. 2012 Nov;43(5):2027-35. doi: 10.1007/s00726-012-1277-z. Epub 2012 Apr 4. Amino Acids. 2012. PMID: 22476347 Free PMC article.
-
3-OH flavone inhibition of epidermal growth factor-induced proliferaton through blocking prostaglandin E2 production.Int J Cancer. 2004 Feb 10;108(4):502-10. doi: 10.1002/ijc.11581. Int J Cancer. 2004. PMID: 14696113
-
[Role and mechanism of the regulation of nuclear factor-κB by heparin binding-epidermal growth factor-like growth factor in the induction of paclitaxel resistance of ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2019 Apr 25;54(4):255-261. doi: 10.3760/cma.j.issn.0529-567x.2019.04.008. Zhonghua Fu Chan Ke Za Zhi. 2019. PMID: 31006192 Chinese.
-
The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21.Cancer Biol Ther. 2010 Jun 1;9(11):928-35. doi: 10.4161/cbt.9.11.11873. Epub 2010 Jun 25. Cancer Biol Ther. 2010. PMID: 20404564 Free PMC article.
Cited by
-
Computational Metabolomics Reveals the Potential Mechanism of Matrine Mediated Metabolic Network Against Hepatocellular Carcinoma.Front Cell Dev Biol. 2022 Jul 22;10:859236. doi: 10.3389/fcell.2022.859236. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938176 Free PMC article.
-
Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature.Int J Mol Sci. 2020 Oct 31;21(21):8169. doi: 10.3390/ijms21218169. Int J Mol Sci. 2020. PMID: 33142915 Free PMC article. Review.
-
Aspirin inhibits the proliferation of hepatoma cells through controlling GLUT1-mediated glucose metabolism.Acta Pharmacol Sin. 2019 Jan;40(1):122-132. doi: 10.1038/s41401-018-0014-x. Epub 2018 Jun 20. Acta Pharmacol Sin. 2019. PMID: 29925918 Free PMC article.
-
Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature.Pharmaceuticals (Basel). 2018 Oct 11;11(4):101. doi: 10.3390/ph11040101. Pharmaceuticals (Basel). 2018. PMID: 30314310 Free PMC article. Review.
-
Aspirin Suppressed PD-L1 Expression through Suppressing KAT5 and Subsequently Inhibited PD-1 and PD-L1 Signaling to Attenuate OC Development.J Oncol. 2022 Mar 29;2022:4664651. doi: 10.1155/2022/4664651. eCollection 2022. J Oncol. 2022. PMID: 35392432 Free PMC article.
References
-
- Chobanian N, Dietrich CS. Ovarian cancer. Surg Clin North Am. 2008;88:285–99. - PubMed
-
- Sudo T. Molecular-targeted therapies for ovarian cancer: prospects for the future. Int J Clin Oncol. 2012;17:424–9. - PubMed
-
- Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58:133–47. - PubMed
-
- Pejovic T, Nezhat F. Missing link: inflammation and ovarian cancer. Lancet Oncol. 2011;12:833–4. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

