Expression of plant sweet protein brazzein in the milk of transgenic mice
- PMID: 24155905
- PMCID: PMC3796561
- DOI: 10.1371/journal.pone.0076769
Expression of plant sweet protein brazzein in the milk of transgenic mice
Abstract
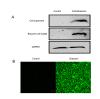
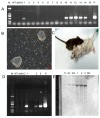
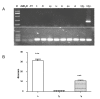
Sugar, the most popular sweetener, is essential in daily food. However, excessive sugar intake has been associated with several lifestyle-related diseases. Finding healthier and more economical alternatives to sugars and artificial sweeteners has received increasing attention to fulfill the growing demand. Brazzein, which comes from the pulp of the edible fruit of the African plant Pentadiplandra brazzeana Baill, is a protein that is 2,000 times sweeter than sucrose by weight. Here we report the production of transgenic mice that carry the optimized brazzein gene driven by the goat Beta-casein promoter, which specifically directs gene expression in the mammary glands. Using western blot analysis and immunohistochemistry, we confirmed that brazzein could be efficiently expressed in mammalian milk, while retaining its sweetness. This study presents the possibility of producing plant protein-sweetened milk from large animals such as cattle and goats.
Conflict of interest statement
Figures



References
-
- Faus I (2000) Recent developments in the characterization and biotechnological production of sweet-tasting proteins. Appl Microbiol Biotechnol 53: 145–151. - PubMed
-
- Ming D, Hellekant G (1994) Brazzein, a new high-potency thermostable sweet protein from Pentadiplandra brazzeana B. FEBS Lett. 355: 106–108. - PubMed
-
- Hellekant G, Danilova V (2005) Brazzein a small, sweet protein: discovery and physiological overview. Chemical senses 30 Suppl 1i88–89. - PubMed
-
- Assadi-Porter FM, Aceti DJ, Cheng H, Markley JL (2000) Efficient production of recombinant brazzein, a small, heat-stable, sweet-tasting protein of plant origin. Archives of biochemistry and biophysics 376: 252–258. - PubMed
-
- Berlec A, Jevnikar Z, Majhenic AC, Rogelj I, Strukelj B (2006) Expression of the sweet-tasting plant protein brazzein in Escherichia coli and Lactococcus lactis: a path toward sweet lactic acid bacteria. Applied microbiology and biotechnology 73: 158–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

