Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome
- PMID: 24155976
- PMCID: PMC3796479
- DOI: 10.1371/journal.pone.0077843
Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome
Abstract
Objective: Dravet syndrome is a severe form of intractable pediatric epilepsy with a high incidence of SUDEP: Sudden Unexpected Death in epilepsy. Cardiac arrhythmias are a proposed cause for some cases of SUDEP, yet the susceptibility and potential mechanism of arrhythmogenesis in Dravet syndrome remain unknown. The majority of Dravet syndrome patients have de novo mutations in SCN1A, resulting in haploinsufficiency. We propose that, in addition to neuronal hyperexcitability, SCN1A haploinsufficiency alters cardiac electrical function and produces arrhythmias, providing a potential mechanism for SUDEP.
Methods: Postnatal day 15-21 heterozygous SCN1A-R1407X knock-in mice, expressing a human Dravet syndrome mutation, were used to investigate a possible cardiac phenotype. A combination of single cell electrophysiology and in vivo electrocardiogram (ECG) recordings were performed.
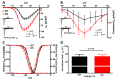
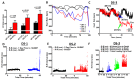
Results: We observed a 2-fold increase in both transient and persistent Na(+) current density in isolated Dravet syndrome ventricular myocytes that resulted from increased activity of a tetrodotoxin-resistant Na(+) current, likely Nav1.5. Dravet syndrome myocytes exhibited increased excitability, action potential duration prolongation, and triggered activity. Continuous radiotelemetric ECG recordings showed QT prolongation, ventricular ectopic foci, idioventricular rhythms, beat-to-beat variability, ventricular fibrillation, and focal bradycardia. Spontaneous deaths were recorded in 2 DS mice, and a third became moribund and required euthanasia.
Interpretation: These data from single cell and whole animal experiments suggest that altered cardiac electrical function in Dravet syndrome may contribute to the susceptibility for arrhythmogenesis and SUDEP. These mechanistic insights may lead to critical risk assessment and intervention in human patients.
Conflict of interest statement
Figures







References
-
- Surges R, Adjei P, Kallis C, Erhuero J, Scott CA et al. (2010) Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia 51: 233-242. doi: 10.1111/j.1528-1167.2009.02330.x. PubMed: 19817816. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

