Ankyrin-G participates in INa remodeling in myocytes from the border zones of infarcted canine heart
- PMID: 24155982
- PMCID: PMC3796465
- DOI: 10.1371/journal.pone.0078087
Ankyrin-G participates in INa remodeling in myocytes from the border zones of infarcted canine heart
Abstract
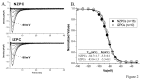
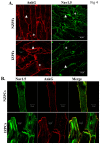
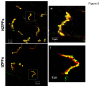
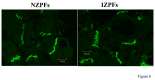
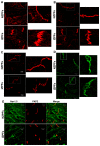
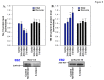
Cardiac Na channel remodeling provides a critical substrate for generation of reentrant arrhythmias in border zones of the infarcted canine heart. Recent studies show that Nav1.5 assembly and function are linked to ankyrin-G, gap, and mechanical junction proteins. In this study our objective is to expound the status of the cardiac Na channel, its interacting protein ankyrinG and the mechanical and gap junction proteins at two different times post infarction when arrhythmias are known to occur; that is, 48 hr and 5 day post coronary occlusion. Previous studies have shown the origins of arrhythmic events come from the subendocardial Purkinje and epicardial border zone. Our Purkinje cell (Pcell) voltage clamp study shows that INa and its kinetic parameters do not differ between Pcells from the subendocardium of the 48hr infarcted heart (IZPCs) and control non-infarcted Pcells (NZPCs). Immunostaining studies revealed that disturbances of Nav1.5 protein location with ankyrin-G are modest in 48 hr IZPCs. Therefore, Na current remodeling does not contribute to the abnormal conduction in the subendocardial border zone 48 hr post myocardial infarction as previously defined. In addition, immunohistochemical data show that Cx40/Cx43 co-localize at the intercalated disc (IDs) of control NZPCs but separate in IZPCs. At the same time, Purkinje cell desmoplakin and desmoglein2 immunostaining become diffuse while plakophilin2 and plakoglobin increase in abundance at IDs. In the epicardial border zone 5 days post myocardial infarction, immunoblot and immunocytochemical analyses showed that ankyrin-G protein expression is increased and re-localized to submembrane cell regions at a time when Nav1.5 function is decreased. Thus, Nav1.5 and ankyrin-G remodeling occur later after myocardial infarction compared to that of gap and mechanical junctional proteins. Gap and mechanical junctional proteins remodel in IZPCs early, perhaps to help maintain Nav1.5 subcellular location position and preserve its function soon after myocardial infarction.
Conflict of interest statement
Figures



References
-
- Wit AL, Janse MJ (1993) The Ventricular Arrhythmias of Ischemia and Infarction. Electrophysiological Mechanisms. Mount Kisco, NY. Futura Publishing Co,Inc.
-
- Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V (2004) Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrinG and expression of NAv1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA 101: 17533-17538. doi:10.1073/pnas.0403711101. PubMed: 15579534. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL084583/HL/NHLBI NIH HHS/United States
- K99 HL096805/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- HL105983/HL/NHLBI NIH HHS/United States
- HL114893/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- HL066140/HL/NHLBI NIH HHS/United States
- HL096805/HL/NHLBI NIH HHS/United States
- R00 HL096805/HL/NHLBI NIH HHS/United States
- R01 HL105983/HL/NHLBI NIH HHS/United States
- R01 HL066140/HL/NHLBI NIH HHS/United States
- R01 HL114893/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

