Functional, metal-based crosslinkers for α-helix induction in short peptides
- PMID: 24156013
- PMCID: PMC3800689
- DOI: 10.1039/C3SC50858G
Functional, metal-based crosslinkers for α-helix induction in short peptides
Abstract
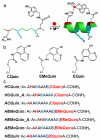
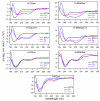
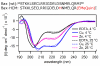
Many protein-protein interactions that play a central role in cellular processes involve α-helical domains. Consequently, there has been great interest in developing strategies for stabilizing short peptides in α-helical conformations toward the inhibition and interrogation of protein-protein interactions. Here, we show that tridentate Hybrid Coordination Motifs (HCMs), which consist of a natural (histidine, His) and an unnatural (8-hydroxyquinoline, Quin) metal binding functionality, can bind divalent metal ions with high affinity and thereby induce/stabilize an α-helical configuration in short peptide sequences. The Quin functionality is readily introduced onto peptide platforms both during or after solid-state peptide synthesis, demonstrating the preparative versatility of HCMs. A systematic study involving a series of HCM-bearing peptides has revealed the critical importance of the length of the linkage between the Quin moiety and the peptide backbone as well as the metal coordination geometry in determining the extent of α-helix induction. Through ZnII coordination or modification with ReI(Quin)(CO)3, the HCM-bearing peptides can be rendered luminescent in the visible region, thus showing that HCMs can be exploited to simultaneously introduce structure and functionality into short peptides.
Figures


Similar articles
-
Tunable Helicity, Stability and DNA-Binding Properties of Short Peptides with Hybrid Metal Coordination Motifs.Chem Sci. 2016 Aug 1;7(8):5453-5461. doi: 10.1039/C6SC00826G. Epub 2016 May 18. Chem Sci. 2016. PMID: 27800151 Free PMC article.
-
Modular and versatile hybrid coordination motifs on alpha-helical protein surfaces.Inorg Chem. 2010 Aug 2;49(15):7106-15. doi: 10.1021/ic100926g. Inorg Chem. 2010. PMID: 20617830 Free PMC article.
-
Linkage isomerism in the binding of pentapeptide Ac-His(Ala)3His-NH2 to (ethylenediamine)palladium(II): effect of the binding mode on peptide conformation.Inorg Chem. 2008 Oct 20;47(20):9439-49. doi: 10.1021/ic800970p. Epub 2008 Sep 13. Inorg Chem. 2008. PMID: 18788796
-
Peptide design using alpha,beta-dehydro amino acids: from beta-turns to helical hairpins.Biopolymers. 2004;76(2):150-61. doi: 10.1002/bip.10571. Biopolymers. 2004. PMID: 15054895 Review.
-
Exploring Helical Peptides and Foldamers for the Design of Metal Helix Frameworks: Current Trends and Future Perspectives.Angew Chem Int Ed Engl. 2023 Feb 1;62(6):e202214583. doi: 10.1002/anie.202214583. Epub 2022 Dec 15. Angew Chem Int Ed Engl. 2023. PMID: 36434750 Review.
Cited by
-
Dual Control of Peptide Conformation with Light and Metal Coordination.Chemistry. 2021 Jun 21;27(35):8956-8959. doi: 10.1002/chem.202101006. Epub 2021 May 21. Chemistry. 2021. PMID: 33909298 Free PMC article.
-
Convergent diversity-oriented side-chain macrocyclization scan for unprotected polypeptides.Org Biomol Chem. 2014 Jan 28;12(4):566-73. doi: 10.1039/c3ob42168f. Org Biomol Chem. 2014. PMID: 24310320 Free PMC article.
-
Tunable Helicity, Stability and DNA-Binding Properties of Short Peptides with Hybrid Metal Coordination Motifs.Chem Sci. 2016 Aug 1;7(8):5453-5461. doi: 10.1039/C6SC00826G. Epub 2016 May 18. Chem Sci. 2016. PMID: 27800151 Free PMC article.
-
A glutamine-based single α-helix scaffold to target globular proteins.Nat Commun. 2022 Nov 18;13(1):7073. doi: 10.1038/s41467-022-34793-6. Nat Commun. 2022. PMID: 36400768 Free PMC article.
-
Folding of unstructured peptoids and formation of hetero-bimetallic peptoid complexes upon side-chain-to-metal coordination.Chem Sci. 2018 Oct 17;10(2):620-632. doi: 10.1039/c8sc03616k. eCollection 2019 Jan 14. Chem Sci. 2018. PMID: 30713653 Free PMC article.
References
-
- Orner BP, Ernst JT, Hamilton AD. J. Am. Chem. Soc. 2001;123:5382. - PubMed
- Seebach D, Matthews JL. Chem. Commun. 1997:2015. 0.
- Gellman SH. Acc. Chem. Res. 1998;31:173.
- Cheng RP, DeGrado WF. J. Am. Chem. Soc. 2001;123:5162. - PubMed
- Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J. Am. Chem. Soc. 2004;126:9468. - PubMed
- Fowler SA, Blackwell HE. Org. Biomol. Chem. 2009;7:1508. - PMC - PubMed
- Appella DH, Christianson LA, Klein DA, Powell DR, Huang X, Barchi JJ, Gellman SH. Nature. 1997;387:381. - PubMed
-
- Sato AK, Viswanathan M, Kent RB, Wood CR. Curr. Opin. Biotechnol. 2006;17:638. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

