Endoscopic probe optics for spectrally encoded confocal microscopy
- PMID: 24156054
- PMCID: PMC3799656
- DOI: 10.1364/BOE.4.001925
Endoscopic probe optics for spectrally encoded confocal microscopy
Abstract
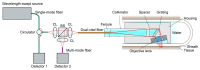
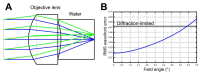
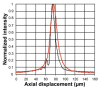
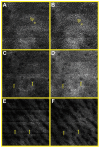
Spectrally encoded confocal microscopy (SECM) is a form of reflectance confocal microscopy that can achieve high imaging speeds using relatively simple probe optics. Previously, the feasibility of conducting large-area SECM imaging of the esophagus in bench top setups has been demonstrated. Challenges remain, however, in translating SECM into a clinically-useable device; the tissue imaging performance should be improved, and the probe size needs to be significantly reduced so that it can fit into luminal organs of interest. In this paper, we report the development of new SECM endoscopic probe optics that addresses these challenges. A custom water-immersion aspheric singlet (NA = 0.5) was developed and used as the objective lens. The water-immersion condition was used to reduce the spherical aberrations and specular reflection from the tissue surface, which enables cellular imaging of the tissue deep below the surface. A custom collimation lens and a small-size grating were used along with the custom aspheric singlet to reduce the probe size. A dual-clad fiber was used to provide both the single- and multi- mode detection modes. The SECM probe optics was made to be 5.85 mm in diameter and 30 mm in length, which is small enough for safe and comfortable endoscopic imaging of the gastrointestinal tract. The lateral resolution was 1.8 and 2.3 µm for the single- and multi- mode detection modes, respectively, and the axial resolution 11 and 17 µm. SECM images of the swine esophageal tissue demonstrated the capability of this device to enable the visualization of characteristic cellular structural features, including basal cell nuclei and papillae, down to the imaging depth of 260 µm. These results suggest that the new SECM endoscopic probe optics will be useful for imaging large areas of the esophagus at the cellular scale in vivo.
Keywords: (170.1790) Confocal microscopy; (170.2150) Endoscopic imaging; (170.2680) Gastrointestinal; (170.4730) Optical pathology.
Figures



References
-
- Kiesslich R., Gossner L., Goetz M., Dahlmann A., Vieth M., Stolte M., Hoffman A., Jung M., Nafe B., Galle P. R., Neurath M. F., “In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy,” Clin. Gastroenterol. Hepatol. 4(8), 979–987 (2006).10.1016/j.cgh.2006.05.010 - DOI - PubMed
-
- Kitabatake S., Niwa Y., Miyahara R., Ohashi A., Matsuura T., Iguchi Y., Shimoyama Y., Nagasaka T., Maeda O., Ando T., Ohmiya N., Itoh A., Hirooka Y., Goto H., “Confocal endomicroscopy for the diagnosis of gastric cancer in vivo,” Endoscopy 38(11), 1110–1114 (2006).10.1055/s-2006-944855 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
