MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis
- PMID: 24156369
- PMCID: PMC3840398
- DOI: 10.1042/AN20130033
MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis
Abstract
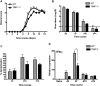
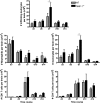
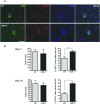
Infection of the CNS (central nervous system) with a sublethal neurotropic coronavirus (JHMV) induces a vigorous inflammatory response. CD4⁺ and CD8⁺ T cells are essential to control infectious virus but at the cost of tissue damage. An enigma in understanding the contribution of T cell subsets in pathogenesis resides in their distinct migration pattern across the BBB (blood brain barrier). CD4⁺ T cells transiently accumulate within the perivascular space, whereas CD8⁺ T cells migrate directly into the CNS parenchyma. As MMPs (matrix metalloproteinases) facilitate migration across the glia limitans, specific expression of the TIMP (tissue inhibitor of MMPs)-1 by CD4⁺ T cells present in the perivascular cuffs suggested that TIMP-1 is responsible for stalling CD4⁺ T cell migration into the CNS parenchyma. Using TIMP-1 deficient mice, the present data demonstrate an increase rather than a decrease in CD4⁺ T cell accumulation within the perivascular space during JHMV infection. Whereas virus control was not affected by perivascular retention of CD4⁺ T cells, disease severity was decreased and associated with reduced IFNγ (interferon γ) production. Moreover, decreased CD4⁺ T cell recruitment into the CNS parenchyma of TIMP-1 deficient mice was not associated with impaired T cell recruiting chemokines or MMP expression, and no compensation by other TIMP molecules was identified. These data suggest an MMP-independent role of TIMP-1 in regulating CD4⁺ T cell access into the CNS parenchyma during acute JHMV encephalitis.
Figures


Similar articles
-
The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model.Viruses. 2023 Dec 9;15(12):2400. doi: 10.3390/v15122400. Viruses. 2023. PMID: 38140641 Free PMC article. Review.
-
T-cell production of matrix metalloproteinases and inhibition of parasite clearance by TIMP-1 during chronic Toxoplasma infection in the brain.ASN Neuro. 2011 Jan 21;3(1):e00049. doi: 10.1042/AN20100027. ASN Neuro. 2011. PMID: 21434872 Free PMC article.
-
Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis.J Virol. 2005 Apr;79(8):4764-73. doi: 10.1128/JVI.79.8.4764-4773.2005. J Virol. 2005. PMID: 15795262 Free PMC article.
-
Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis.J Virol. 2010 May;84(10):4878-88. doi: 10.1128/JVI.00051-10. Epub 2010 Mar 3. J Virol. 2010. PMID: 20200240 Free PMC article.
-
Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease.Viral Immunol. 2019 Jan/Feb;32(1):25-37. doi: 10.1089/vim.2018.0073. Epub 2018 Aug 15. Viral Immunol. 2019. PMID: 30109979 Free PMC article. Review.
Cited by
-
EP4 Antagonist-Elicited Extracellular Vesicles from Mesenchymal Stem Cells Rescue Cognition/Learning Deficiencies by Restoring Brain Cellular Functions.Stem Cells Transl Med. 2019 Jul;8(7):707-723. doi: 10.1002/sctm.18-0284. Epub 2019 Mar 19. Stem Cells Transl Med. 2019. PMID: 30891948 Free PMC article.
-
RNA-Seq Reveals Different Gene Expression in Liver-Specific Prohibitin 1 Knock-Out Mice.Front Physiol. 2021 Sep 3;12:717911. doi: 10.3389/fphys.2021.717911. eCollection 2021. Front Physiol. 2021. PMID: 34539442 Free PMC article.
-
The TIMP protein family: diverse roles in pathophysiology.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C917-C934. doi: 10.1152/ajpcell.00699.2023. Epub 2024 Jan 29. Am J Physiol Cell Physiol. 2024. PMID: 38284123 Free PMC article. Review.
-
The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model.Viruses. 2023 Dec 9;15(12):2400. doi: 10.3390/v15122400. Viruses. 2023. PMID: 38140641 Free PMC article. Review.
-
SOD3 induces a HIF-2α-dependent program in endothelial cells that provides a selective signal for tumor infiltration by T cells.J Immunother Cancer. 2020 Jun;8(1):e000432. doi: 10.1136/jitc-2019-000432. J Immunother Cancer. 2020. PMID: 32591431 Free PMC article.
References
-
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. - PubMed
-
- Althoff GE, Wolfer DP, Timmesfeld N, Kanzler B, Schrewe H, Pagenstecher A. Long-term expression of tissue-inhibitor of matrix metalloproteinase-1 in the murine central nervous system does not alter the morphological and behavioral phenotype but alleviates the course of experimental allergic encephalomyelitis. Am J Pathol. 2010;177:840–853. - PMC - PubMed
-
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

