Pubertal high fat diet: effects on mammary cancer development
- PMID: 24156623
- PMCID: PMC3978633
- DOI: 10.1186/bcr3561
Pubertal high fat diet: effects on mammary cancer development
Abstract
Introduction: Epidemiological studies linking dietary fat intake and obesity to breast cancer risk have produced inconsistent results. This may be due to the difficulty of dissociating fat intake from obesity, and/or the lack of defined periods of exposure in these studies. The pubertal mammary gland is highly sensitive to cancer-causing agents. We assessed how high fat diet (HFD) affects inflammation, proliferative, and developmental events in the pubertal gland, since dysregulation of these can promote mammary tumorigenesis. To test the effect of HFD initiated during puberty on tumorigenesis, we utilized BALB/c mice, for which HFD neither induces obesity nor metabolic syndrome, allowing dissociation of HFD effects from other conditions associated with HFD.
Methods: Pubertal BALB/c mice were fed a low fat diet (12% kcal fat) or a HFD (60% kcal fat), and subjected to carcinogen 7,12-dimethylbenz[a]anthracene (DMBA)-induced tumorigenesis.
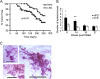
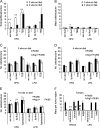
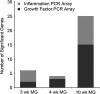
Results: HFD elevated mammary gland expression of inflammatory and growth factor genes at 3 and 4 weeks of diet. Receptor activator of nuclear factor kappa-B ligand (RANKL), robustly induced at 4 weeks, has direct mitogenic activity in mammary epithelial cells and, as a potent inducer of NF-κB activity, may induce inflammatory genes. Three weeks of HFD induced a transient influx of eosinophils into the mammary gland, consistent with elevated inflammatory factors. At 10 weeks, prior to the appearance of palpable tumors, there were increased numbers of abnormal mammary epithelial lesions, enhanced cellular proliferation, increased growth factors, chemokines associated with immune-suppressive regulatory T cells, increased vascularization, and elevated M2 macrophages. HFD dramatically reduced tumor latency. Early developing tumors were more proliferative and were associated with increased levels of tumor-related growth factors, including increased plasma levels of HGF in tumor-bearing animals. Early HFD tumors also had increased vascularization, and more intra-tumor and stromal M2 macrophages.
Conclusions: Taken together in this non-obesogenic context, HFD promotion of inflammatory processes, as well as local and systemically increased growth factor expression, are likely responsible for the enhanced tumorigenesis. It is noteworthy that although DMBA mutagenesis is virtually random in its targeting of genes in tumorigenesis, the short latency tumors arising in animals on HFD showed a unique gene expression profile, highlighting the potent overarching influence of HFD.
Figures
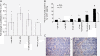
Similar articles
-
Puberty-specific promotion of mammary tumorigenesis by a high animal fat diet.Breast Cancer Res. 2015 Nov 2;17(1):138. doi: 10.1186/s13058-015-0646-4. Breast Cancer Res. 2015. PMID: 26526858 Free PMC article.
-
Pubertal and adult windows of susceptibility to a high animal fat diet in Trp53-null mammary tumorigenesis.Oncotarget. 2016 Dec 13;7(50):83409-83423. doi: 10.18632/oncotarget.13112. Oncotarget. 2016. PMID: 27825136 Free PMC article.
-
Pubertally Initiated High-Fat Diet Promotes Mammary Tumorigenesis in Obesity-Prone FVB Mice Similarly to Obesity-Resistant BALB/c Mice.Transl Oncol. 2017 Dec;10(6):928-935. doi: 10.1016/j.tranon.2017.09.004. Epub 2017 Oct 9. Transl Oncol. 2017. PMID: 29024822 Free PMC article.
-
Timing of dietary fat exposure and mammary tumorigenesis: role of estrogen receptor and protein kinase C activity.Mol Cell Biochem. 1998 Nov;188(1-2):5-12. Mol Cell Biochem. 1998. PMID: 9823005 Review.
-
Dietary fat, calories, and mammary gland tumorigenesis.Adv Exp Med Biol. 1992;322:203-22. doi: 10.1007/978-1-4684-7953-9_16. Adv Exp Med Biol. 1992. PMID: 1442296 Review.
Cited by
-
Chemically induced carcinogenesis in rodent models of aging: assessing organismal resilience to genotoxic stressors in geroscience research.Geroscience. 2019 Apr;41(2):209-227. doi: 10.1007/s11357-019-00064-4. Epub 2019 Apr 29. Geroscience. 2019. PMID: 31037472 Free PMC article. Review.
-
Puberty is a critical window for the impact of diet on mammary gland development in the rabbit.Dev Dyn. 2019 Oct;248(10):948-960. doi: 10.1002/dvdy.91. Epub 2019 Aug 2. Dev Dyn. 2019. PMID: 31348557 Free PMC article.
-
Mammary gland development--It's not just about estrogen.J Dairy Sci. 2016 Jan;99(1):875-83. doi: 10.3168/jds.2015-10105. Epub 2015 Oct 23. J Dairy Sci. 2016. PMID: 26506542 Free PMC article. Review.
-
High-fat circulating nutrients promote growth and invasion in a 3D microfluidic tumor model of triple-negative breast cancer.bioRxiv [Preprint]. 2025 Jul 16:2025.07.10.664224. doi: 10.1101/2025.07.10.664224. bioRxiv. 2025. PMID: 40791523 Free PMC article. Preprint.
-
The role of obesity and bariatric surgery-induced weight loss in breast cancer.Cancer Metastasis Rev. 2022 Sep;41(3):673-695. doi: 10.1007/s10555-022-10050-6. Epub 2022 Jul 23. Cancer Metastasis Rev. 2022. PMID: 35870055 Free PMC article. Review.
References
-
- van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. et al.Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;15:514–527. doi: 10.1093/aje/152.6.514. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

