Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage
- PMID: 24156724
- PMCID: PMC3815232
- DOI: 10.1186/1471-2202-14-131
Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage
Abstract
Background: Endothelin-1 (ET-1) is a potent vasoconstrictor, and astrocytic ET-1 is reported to play a role in the pathogenesis of cerebral ischemic injury and cytotoxic edema. However, it is still unknown whether astrocytic ET-1 also contributes to vasogenic edema and vasospasm during subarachnoid hemorrhage (SAH). In the present study, transgenic mice with astrocytic endothelin-1 over-expression (GET-1 mice) were used to investigate the pathophysiological role of ET-1 in SAH pathogenesis.
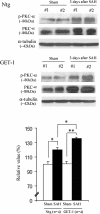
Results: The GET-1 mice experienced a higher mortality rate and significantly more severe neurological deficits, blood-brain barrier breakdown and vasogenic edema compared to the non-transgenic (Ntg) mice following SAH. Oral administration of vasopressin V1a receptor antagonist, SR 49059, significantly reduced the cerebral water content in the GET-1 mice. Furthermore, the GET-1 mice showed significantly more pronounced middle cerebral arterial (MCA) constriction after SAH. Immunocytochemical analysis showed that the calcium-activated potassium channels and the phospho-eNOS were significantly downregulated, whereas PKC-α expression was significantly upregulated in the MCA of the GET-1 mice when compared to Ntg mice after SAH. Administration of ABT-627 (ETA receptor antagonist) significantly down-regulated PKC-α expression in the MCA of the GET-1 mice following SAH.
Conclusions: The present study suggests that astrocytic ET-1 involves in SAH-induced cerebral injury, edema and vasospasm, through ETA receptor and PKC-mediated potassium channel dysfunction. Administration of ABT-627 (ETA receptor antagonist) and SR 49059 (vasopressin V1a receptor antagonist) resulted in amelioration of edema and vasospasm in mice following SAH. These data provide a strong rationale to investigate SR 49059 and ABT-627 as therapeutic drugs for the treatment of SAH patients.
Figures





Similar articles
-
Role of Cyclooxygenase-2 in Relation to Nitric Oxide and Endothelin-1 on Pathogenesis of Cerebral Vasospasm After Subarachnoid Hemorrhage in Rabbit.Transl Stroke Res. 2016 Jun;7(3):220-7. doi: 10.1007/s12975-016-0466-6. Epub 2016 Apr 5. Transl Stroke Res. 2016. PMID: 27044361
-
Influence endothelin ETA receptor antagonist--BQ-123--on changes of endothelin-1 level in plasma of rats with acute vasospasm following subarachnoid hemorrhage.J Physiol Pharmacol. 1998 Sep;49(3):367-75. J Physiol Pharmacol. 1998. PMID: 9789790
-
Microvasospasms After Experimental Subarachnoid Hemorrhage Do Not Depend on Endothelin A Receptors.Stroke. 2018 Mar;49(3):693-699. doi: 10.1161/STROKEAHA.117.020028. Epub 2018 Feb 8. Stroke. 2018. PMID: 29438081
-
Systemic administration of the endothelin-A receptor antagonist TBC 11251 attenuates cerebral vasospasm after experimental subarachnoid hemorrhage: dose study and review of endothelin-based therapies in the literature on cerebral vasospasm.Neurosurgery. 1998 Dec;43(6):1409-17; discussion 1417-8. Neurosurgery. 1998. PMID: 9848855 Review.
-
Subarachnoid haemorrhage: what happens to the cerebral arteries?Clin Exp Pharmacol Physiol. 1998 Nov;25(11):867-76. doi: 10.1111/j.1440-1681.1998.tb02337.x. Clin Exp Pharmacol Physiol. 1998. PMID: 9807657 Review.
Cited by
-
Angiogenesis Biomarkers in Ischemic Stroke Patients.J Inflamm Res. 2021 Sep 22;14:4893-4900. doi: 10.2147/JIR.S331868. eCollection 2021. J Inflamm Res. 2021. PMID: 34588795 Free PMC article.
-
The role of inflammation and potential use of sex steroids in intracranial aneurysms and subarachnoid hemorrhage.Surg Neurol Int. 2018 Jul 26;9:150. doi: 10.4103/sni.sni_88_18. eCollection 2018. Surg Neurol Int. 2018. PMID: 30105144 Free PMC article. Review.
-
The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments.Fluids Barriers CNS. 2022 Apr 11;19(1):29. doi: 10.1186/s12987-022-00312-4. Fluids Barriers CNS. 2022. PMID: 35410231 Free PMC article. Review.
-
Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview.Pharmaceutics. 2021 Oct 26;13(11):1779. doi: 10.3390/pharmaceutics13111779. Pharmaceutics. 2021. PMID: 34834200 Free PMC article. Review.
-
The harmful effects of subarachnoid hemorrhage on extracerebral organs.Biomed Res Int. 2014;2014:858496. doi: 10.1155/2014/858496. Epub 2014 Jul 7. Biomed Res Int. 2014. PMID: 25110700 Free PMC article. Review.
References
-
- Macdonald RL. Pathophysiology and molecular genetics of vasospasm. Acta Neurochir Suppl. 2001;77:7–11. - PubMed
-
- Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit Care. 2010;12(1):124–131. doi: 10.1007/s12028-009-9277-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

