Enantioselective functionalization of allylic C-H bonds following a strategy of functionalization and diversification
- PMID: 24156776
- PMCID: PMC3911985
- DOI: 10.1021/ja409995w
Enantioselective functionalization of allylic C-H bonds following a strategy of functionalization and diversification
Abstract
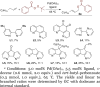
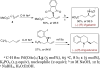
We report the enantioselective functionalization of allylic C-H bonds in terminal alkenes by a strategy involving the installation of a temporary functional group at the terminal carbon atom by C-H bond functionalization, followed by the catalytic diversification of this intermediate with a broad scope of reagents. The method consists of a one-pot sequence of palladium-catalyzed allylic C-H bond oxidation under neutral conditions to form linear allyl benzoates, followed by iridium-catalyzed allylic substitution. This overall transformation forms a variety of chiral products containing a new C-N, C-O, C-S, or C-C bond at the allylic position in good yield with a high branched-to-linear selectivity and excellent enantioselectivity (ee ≤97%). The broad scope of the overall process results from separating the oxidation and functionalization steps; by doing so, the scope of nucleophile encompasses those sensitive to direct oxidative functionalization. The high enantioselectivity of the overall process is achieved by developing an allylic oxidation that occurs without acid to form the linear isomer with high selectivity. These allylic functionalization processes are amenable to an iterative sequence leading to (1,n)-functionalized products with catalyst-controlled diastereo- and enantioselectivity. The utility of the method in the synthesis of biologically active molecules has been demonstrated.
Figures



Similar articles
-
Enantioselective Allylic C-H Oxidation of Terminal Olefins to Isochromans by Palladium(II)/Chiral Sulfoxide Catalysis.Angew Chem Int Ed Engl. 2016 Aug 8;55(33):9571-5. doi: 10.1002/anie.201603576. Epub 2016 Jul 4. Angew Chem Int Ed Engl. 2016. PMID: 27376625 Free PMC article.
-
Iridium-catalyzed kinetic asymmetric transformations of racemic allylic benzoates.J Am Chem Soc. 2010 Jul 7;132(26):8918-20. doi: 10.1021/ja103779e. J Am Chem Soc. 2010. PMID: 20552969 Free PMC article.
-
Palladium- or iridium-catalyzed allylic substitution of guanidines: convenient and direct modification of guanidines.J Org Chem. 2009 Jan 2;74(1):305-11. doi: 10.1021/jo802271d. J Org Chem. 2009. PMID: 19053613
-
Palladium-catalyzed allylic C-H bond functionalization of olefins.Top Curr Chem. 2010;292:195-209. doi: 10.1007/128_2009_16. Top Curr Chem. 2010. PMID: 21500407 Review.
-
Recent advances and applications of iridium-catalysed asymmetric allylic substitution.Org Biomol Chem. 2012 Apr 28;10(16):3147-63. doi: 10.1039/c2ob07086c. Epub 2012 Mar 12. Org Biomol Chem. 2012. PMID: 22407450 Review.
Cited by
-
A site-selective amination catalyst discriminates between nearly identical C-H bonds of unsymmetrical disubstituted alkenes.Nat Chem. 2020 Aug;12(8):725-731. doi: 10.1038/s41557-020-0470-z. Epub 2020 Jun 15. Nat Chem. 2020. PMID: 32541949 Free PMC article.
-
O2-Promoted Allylic Acetoxylation of Alkenes: Assessment of "Push" vs. "Pull" Mechanisms and Comparison between O2 and Benzoquinone.Polyhedron. 2014 Dec 14;84:96-102. doi: 10.1016/j.poly.2014.06.038. Polyhedron. 2014. PMID: 25435646 Free PMC article.
-
Structurally Diverse Diazafluorene-Ligated Palladium(II) Complexes and Their Implications for Aerobic Oxidation Reactions.J Am Chem Soc. 2016 Apr 13;138(14):4869-80. doi: 10.1021/jacs.6b01188. Epub 2016 Apr 5. J Am Chem Soc. 2016. PMID: 26967703 Free PMC article.
-
Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives.Angew Chem Int Ed Engl. 2018 Feb 19;57(8):2233-2237. doi: 10.1002/anie.201710915. Epub 2018 Jan 18. Angew Chem Int Ed Engl. 2018. PMID: 29232488 Free PMC article.
-
Copper-catalyzed oxidative dehydrogenative carboxylation of unactivated alkanes to allylic esters via alkenes.J Am Chem Soc. 2014 Dec 10;136(49):17292-301. doi: 10.1021/ja510093x. Epub 2014 Nov 24. J Am Chem Soc. 2014. PMID: 25389772 Free PMC article.
References
-
- Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752. - PubMed
-
- White MC. Science. 2012;335:807. - PubMed
- Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem., Int. Ed. 2012;51:8960. - PubMed
- Song GY, Wang F, Li XW. Chem. Soc. Rev. 2012;41:3651. - PubMed
- Ramirez TA, Zhao BG, Shi Y. Chem. Soc. Rev. 2012;41:931. - PubMed
- Neufeldt SR, Sanford MS. Acc. Chem. Res. 2012;45:936. - PMC - PubMed
- Roizen JL, Harvey ME, Du Bois J. Acc. Chem. Res. 2012;45:911. - PMC - PubMed
- Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Angew. Chem., Int. Ed. 2012;51:10236. - PubMed
- Campbell AN, Stahl SS. Acc. Chem. Res. 2012;45:851. - PMC - PubMed
- McMurray L, O'Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885. - PubMed
- Boorman TC, Larrosa I. Chem. Soc. Rev. 2011;40:1910. - PubMed
- Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. - PubMed
- Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. - PMC - PubMed
- Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem. Soc. Rev. 2009;38:3242. - PubMed
- Bergman RG. Nature. 2007;446:391. - PubMed
- Sun CL, Li BJ, Shi ZJ. Chem. Rev. 2011;111:1293. - PubMed
-
- El-Qisiari AK, Qaseer HA, Henry PM. Tetrahedron Lett. 2002;43:4229.
- Clark JS, Roche C. Chem. Commun. 2005:5175. - PubMed
- Zhou ZN, Andrus MB. Tetrahedron Lett. 2012;53:4518.
- Hoang VDM, Reddy PAN, Kim TJ. Organometallics. 2008;27:1026.
- Eames J, Watkinson M. Angew. Chem., Int. Ed. 2001;40:3567. - PubMed
- Malkov AV, Pernazza D, Bell M, Bella M, Massa A, Teply F, Meghani P, Kocovsky P. J. Org. Chem. 2003;68:4727. - PubMed
- Andrus MB, Zhou ZN. J. Am. Chem. Soc. 2002;124:8806. - PubMed
- Chai Z, Rainey TJ. J. Am. Chem. Soc. 2012;134:3615. - PubMed
- Li QA, Yu ZX. Angew. Chem. 2011;50:2144. - PubMed
- Collet F, Lescot C, Dauban P. Chem. Soc. Rev. 2011;40:1926. - PubMed
- Covell DJ, White MC. Angew. Chem., Int. Ed. 2008;47:6448. - PMC - PubMed
- Nishioka Y, Uchida T, Katsuki T. Angew. Chem., Int. Ed. 2013;52:1739. - PubMed
- Trost BM, Thaisrivongs DA, Donckele JE. Angew. Chem., Int. Ed. 2012;52:1523. - PubMed
- Davies HML, Morton D. Chem. Soc. Rev. 2011;40:1857. - PubMed
- Bao HL, Tambar UK. J. Am. Chem. Soc. 2012;134:18495. - PMC - PubMed
-
- Hartwig JF. Organotransition metal chemistry : from bonding to catalysis. University Science Books; Sausalito, Calif: 2010.
- Tsuji J. In: Palladium reagents and catalysts : new perspectives for the 21st century. 2nd ed. Wiley J, editor. Chichester, West Sussex; Hoboken, NJ: 2004.
- Buchwald SL. Adv. Synth. Catal. 2004;346:1524. - PMC - PubMed
- March J. Advanced organic chemistry: reactions, mechanisms, and structure. McGraw-Hill; New York: 1968.
-
- Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. - PMC - PubMed
- Dai HX, Yu JQ. J. Am. Chem. Soc. 2012;134:134. - PubMed
- Wang CM, Chen H, Wang ZF, Chen JA, Huang Y. Angew. Chem., Int. Ed. 2012;51:7242. - PubMed
- Rousseau G, Breit B. Angew. Chem., Int. Ed. 2011;50:2450. - PubMed
- Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

