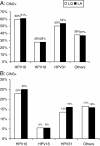
Comparison of the performance of carcinogenic HPV typing of the Roche Linear Array and Qiagen LiquiChip® HPV assays
- PMID: 24156822
- PMCID: PMC4015444
- DOI: 10.1186/1471-2334-13-499
Comparison of the performance of carcinogenic HPV typing of the Roche Linear Array and Qiagen LiquiChip® HPV assays
Abstract
Background: Cervical cancer is caused by high-risk types of human papillomavirus (HPV). DNA testing of such high-risk types of HPV could improve cervical screening.The aim of the study was to compare the sensitivities and positive predictive values of two commercially available typing assays (Qiagen LQ and Roche LA) and to comparatively assess the distribution of HPV types with these two assays.
Methods: The study population comprised 311 ASCUS + women with abnormal pap tests who were HCII positive and who were admitted to three European referral gynecology clinics between 2007 and 2010 (Madrid, Marseille and Milan). All patients underwent LQ and LA tests.
Results: The sensitivity of the two assays for HPV typing was 94% for LQ and 99% for LA (compared with HCII). The overall concordance between LQ and LA was 93%. The three prevalent genotypes, HPV16, HPV18, and HPV31, were identified with a high concordance using the two assays: kappa 0.93, 0.83, and 0.91, respectively. Mixed genotypes were more frequently detected by LA than by LQ: 52% vs. 18%, respectively (p < .0001).
Conclusions: These assays have a good clinical sensitivity for detecting HPV types in CIN2+ patients and allow the virus type to be detected in the same experiment. Our study revealed no significant difference between LQ and LA for CIN2+ or CIN3+ diagnosis, indicating similar distributions of HPV types and a mixed genotype detection that is higher for LA than for LQ.
Figures
Similar articles
-
Comparison of the clinical performance of carcinogenic HPV typing of the Linear Array and Papillocheck HPV-screening assay.J Clin Virol. 2010 Jan;47(1):38-42. doi: 10.1016/j.jcv.2009.10.013. Epub 2009 Nov 25. J Clin Virol. 2010. PMID: 19939732
-
Evaluation of the clinical performance of the Abbott RealTime High-Risk HPV for carcinogenic HPV detection.J Clin Virol. 2010 Aug;48(4):246-50. doi: 10.1016/j.jcv.2010.05.008. Epub 2010 Jun 11. J Clin Virol. 2010. PMID: 20541966
-
Assessment of the Roche Linear Array HPV Genotyping Test within the VALGENT framework.J Clin Virol. 2018 Jan;98:37-42. doi: 10.1016/j.jcv.2017.12.001. Epub 2017 Dec 6. J Clin Virol. 2018. PMID: 29241150
-
Relevance of HPV mRNA detection in a population of ASCUS plus women using the NucliSENS EasyQ HPV assay.J Clin Virol. 2010 Feb;47(2):177-81. doi: 10.1016/j.jcv.2009.11.011. Epub 2009 Dec 22. J Clin Virol. 2010. PMID: 20022804
-
Comparison of linear array and line blot assay for detection of human papillomavirus and diagnosis of cervical precancer and cancer in the atypical squamous cell of undetermined significance and low-grade squamous intraepithelial lesion triage study.J Clin Microbiol. 2008 Jan;46(1):109-17. doi: 10.1128/JCM.01667-07. Epub 2007 Nov 7. J Clin Microbiol. 2008. PMID: 17989194 Free PMC article.
Cited by
-
Prevalence of human papilloma virus (HPV) and its genotypes in cervical specimens of Egyptian women by linear array HPV genotyping test.Infect Agent Cancer. 2016 Feb 17;11:6. doi: 10.1186/s13027-016-0053-1. eCollection 2016. Infect Agent Cancer. 2016. PMID: 26889206 Free PMC article.
References
-
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, Dillner J, Meijer CJ. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;13(Suppl 10):K29–K41. - PubMed
-
- Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, Rush BB, Glass AG, Schiffman M. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;13(14):1072–1079. doi: 10.1093/jnci/dji187. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials