The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor (nAChR) ligands. Part 1: the influence of different hydrogen bond acceptor systems on alkyl and (hetero)aryl substituents
- PMID: 24156938
- PMCID: PMC4519239
- DOI: 10.1016/j.bmc.2013.09.059
The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor (nAChR) ligands. Part 1: the influence of different hydrogen bond acceptor systems on alkyl and (hetero)aryl substituents
Abstract
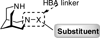
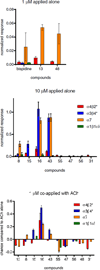
3,7-Diazabicyclo[3.3.1]nonane is a naturally occurring scaffold interacting with nicotinic acetylcholine receptors (nAChRs). When one nitrogen of the 3,7-diazabicyclo[3.3.1]nonane scaffold was implemented in a carboxamide motif displaying a hydrogen bond acceptor (HBA) functionality, compounds with higher affinities and subtype selectivity for α4β2(∗) were obtained. The nature of the HBA system (carboxamide, sulfonamide, urea) had a strong impact on nAChR interaction. High affinity ligands for α4β2(∗) possessed small alkyl chains, small un-substituted hetero-aryl groups or para-substituted phenyl ring systems along with a carboxamide group. Electrophysiological responses of selected 3,7-diazabicyclo[3.3.1]nonane derivatives to Xenopus oocytes expressing various nAChR subtypes showed diverse activation profiles. Compounds with strongest agonistic profiles were obtained with small alkyl groups whereas a shift to partial agonism/antagonism was observed for aryl substituents.
Keywords: 1,2-dimethoxyethane; 3,7-Diazabicyclo[3.3.1]nonane; 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid; 4-(dimethylamino)pyridine; BBB; Bispidine; CD(3)OD; CDCl(3); CH(2)Cl(2); CNS; D(2)O; DCC; DMAP; DME; DMF; Et(2)O; Et(3)N; EtOAc; HBA; HCl; HEPES; HEPES-buffered salt solution; HSS; K(2)CO(3); KBr; KMnO(4); KOH; MeCN; MeI; MeOH; MgSO(4); N,N-dimethylformamide; N,N′-dicyclohexylcarbodiimide; NaHCO(3); NaOH; Nicotinic acetylcholine receptor; PE; PEI; Pd/C; Ro5; Structure–activity relationship; THF; TPSA; TRIS; ZnBr(2); acetonitrile; blood–brain barrier; central nervous system; deuterium oxide; deuterochloroform; dichloromethane; diethyl ether; ethyl acetate; hydrogen bond acceptor; hydrogen chloride; iodomethane; magnesium sulfate; methanol; nAChR; nicotinic acetylcholine receptor; palladium on activated charcoal; petroleum ether; poly(ethyleneimine); potassium bromide; potassium carbonate; potassium hydroxide; potassium permanganate; rule of five; sodium hydrogen carbonate; sodium hydroxide; tetradeuteromethanol; tetrahydrofuran; topological polar surface area; tri(hydroxymethyl)aminomethane; triethylamine; zinc bromide.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor ligands. Part 2: carboxamide derivatives with different spacer motifs.Bioorg Med Chem. 2013 Dec 1;21(23):7309-29. doi: 10.1016/j.bmc.2013.09.060. Epub 2013 Oct 5. Bioorg Med Chem. 2013. PMID: 24145137 Free PMC article.
-
Synthesis and nicotinic receptor activity of chemical space analogues of N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide (PNU-282,987) and 1,4-diazabicyclo[3.2.2]nonane-4-carboxylic acid 4-bromophenyl ester (SSR180711).J Med Chem. 2012 May 24;55(10):4605-18. doi: 10.1021/jm300030r. Epub 2012 May 16. J Med Chem. 2012. PMID: 22591063
-
New quinoline derivatives as nicotinic receptor modulators.Eur J Med Chem. 2016 Mar 3;110:246-58. doi: 10.1016/j.ejmech.2016.01.025. Epub 2016 Jan 19. Eur J Med Chem. 2016. PMID: 26840365
-
Allosteric modulators of the α4β2 subtype of neuronal nicotinic acetylcholine receptors.Biochem Pharmacol. 2011 Oct 15;82(8):952-8. doi: 10.1016/j.bcp.2011.04.020. Epub 2011 May 7. Biochem Pharmacol. 2011. PMID: 21596025 Free PMC article. Review.
-
Natural compounds interacting with nicotinic acetylcholine receptors: from low-molecular weight ones to peptides and proteins.Toxins (Basel). 2015 May 14;7(5):1683-701. doi: 10.3390/toxins7051683. Toxins (Basel). 2015. PMID: 26008231 Free PMC article. Review.
Cited by
-
A Promising PET Tracer for Imaging of α₇ Nicotinic Acetylcholine Receptors in the Brain: Design, Synthesis, and in Vivo Evaluation of a Dibenzothiophene-Based Radioligand.Molecules. 2015 Oct 9;20(10):18387-421. doi: 10.3390/molecules201018387. Molecules. 2015. PMID: 26473809 Free PMC article.
-
The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor ligands. Part 2: carboxamide derivatives with different spacer motifs.Bioorg Med Chem. 2013 Dec 1;21(23):7309-29. doi: 10.1016/j.bmc.2013.09.060. Epub 2013 Oct 5. Bioorg Med Chem. 2013. PMID: 24145137 Free PMC article.
-
The twin drug approach for novel nicotinic acetylcholine receptor ligands.Bioorg Med Chem. 2015 Aug 1;23(15):4375-4389. doi: 10.1016/j.bmc.2015.06.034. Epub 2015 Jun 20. Bioorg Med Chem. 2015. PMID: 26142318 Free PMC article.
-
Highly Selective Synthesis of 6-Glyoxylamidoquinoline Derivatives via Palladium-Catalyzed Aminocarbonylation.Molecules. 2021 Dec 21;27(1):4. doi: 10.3390/molecules27010004. Molecules. 2021. PMID: 35011236 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

