The hammerhead ribozyme: structure, catalysis, and gene regulation
- PMID: 24156940
- PMCID: PMC4008931
- DOI: 10.1016/B978-0-12-381286-5.00001-9
The hammerhead ribozyme: structure, catalysis, and gene regulation
Abstract
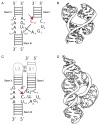
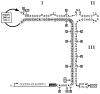
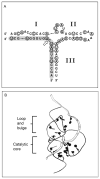
The hammerhead ribozyme has long been considered a prototype for understanding RNA catalysis, but discrepancies between the earlier crystal structures of a minimal hammerhead self-cleaving motif and various biochemical investigations frustrated attempt to understand hammerhead ribozyme catalysis in terms of structure. With the discovery that a tertiary contact distal from the ribozyme's active site greatly enhances its catalytic prowess, and the emergence of new corresponding crystal structures of full-length hammerhead ribozymes, a unified understanding of catalysis in terms of the structure is now possible. A mechanism in which the invariant residue G12 functions as a general base, and the 2'-OH moiety of the invariant G8, itself forming a tertiary base pair with the invariant C3, is the general acid, appears consistent with both the crystal structure and biochemical experimental results. Originally discovered in the context of plant satellite RNA viruses, the hammerhead more recently has been found embedded in the 3'-untranslated region of mature mammalian mRNAs, suggesting additional biological roles in genetic regulation.
Keywords: Catalysis; Gene regulation; Hammerhead ribozyme; RNA; Riboswitch.
© 2013 Elsevier Inc. All rights reserved.
Figures
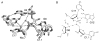
References
-
- Kruger K, Grabowski PJ, Zaug AJ, et al. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. - PubMed
-
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. - PubMed
-
- Prody GA, Bakos JT, Buzayan JM, et al. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986;231:1577–1580. - PubMed
-
- Noller HF, Hoffarth V, Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992;256:1416–1419. - PubMed
-
- Nissen P, Hansen J, Ban N, et al. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000;289:920–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

