Tgfβ-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves
- PMID: 24157418
- PMCID: PMC3869408
- DOI: 10.1016/j.yjmcc.2013.10.007
Tgfβ-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves
Abstract
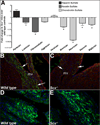
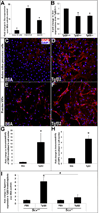
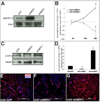
Mature heart valves are complex structures consisting of three highly organized extracellular matrix layers primarily composed of collagens, proteoglycans and elastin. Collectively, these diverse matrix components provide all the necessary biomechanical properties for valve function throughout life. In contrast to healthy valves, myxomatous valve disease is the most common cause of mitral valve prolapse in the human population and is characterized by an abnormal abundance of proteoglycans within the valve tri-laminar structure. Despite the clinical significance, the etiology of this phenotype is not known. Scleraxis (Scx) is a basic-helix-loop-helix transcription factor that we previously showed to be required for establishing heart valve structure during remodeling stages of valvulogenesis. In this study, we report that remodeling heart valves from Scx null mice express decreased levels of proteoglycans, particularly chondroitin sulfate proteoglycans (CSPGs), while overexpression in embryonic avian valve precursor cells and adult porcine valve interstitial cells increases CSPGs. Using these systems we further identify that Scx is positively regulated by canonical Tgfβ2 signaling during this process and this is attenuated by MAPK activity. Finally, we show that Scx is increased in myxomatous valves from human patients and mouse models, and overexpression in human mitral valve interstitial cells modestly increases proteoglycan expression consistent with myxomatous mitral valve phenotypes. Together, these studies identify an important role for Scx in regulating proteoglycans in embryonic and mature valve cells and suggest that imbalanced regulation could influence myxomatous pathogenesis.
Keywords: Heart valve; MAPK; Myxomatous; Proteoglycan; Scleraxis; Tgfβ.
© 2013.
Conflict of interest statement
Figures



References
-
- Lincoln J, Yutzey KE. Molecular and developmental mechanisms of congenital heart valve disease. Birth defects research Part A, Clinical and molecular teratology. 2011;91:526–534. - PubMed
-
- Guy TS, Hill AC. Mitral valve prolapse. Annual review of medicine. 2012;63:277–292. - PubMed
-
- Nasuti JF, Zhang PJ, Feldman MD, Pasha T, Khurana JS, Gorman JH, 3rd, et al. Fibrillin and other matrix proteins in mitral valve prolapse syndrome. The Annals of thoracic surgery. 2004;77:532–536. - PubMed
-
- Akhtar S, Meek KM, James V. Ultrastructure abnormalities in proteoglycans, collagen fibrils, and elastic fibers in normal and myxomatous mitral valve chordae tendineae. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 1999;8:191–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

