Studying immunity to zoonotic diseases in the natural host - keeping it real
- PMID: 24157573
- PMCID: PMC7098194
- DOI: 10.1038/nri3551
Studying immunity to zoonotic diseases in the natural host - keeping it real
Abstract
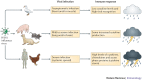
Zoonotic viruses that emerge from wildlife and domesticated animals pose a serious threat to human and animal health. In many instances, mouse models have improved our understanding of the human immune response to infection; however, when dealing with emerging zoonotic diseases, they may be of limited use. This is particularly the case when the model fails to reproduce the disease status that is seen in the natural reservoir, transmission species or human host. In this Review, we discuss how researchers are placing more emphasis on the study of the immune response to zoonotic infections in the natural reservoir hosts and spillover species. Such studies will not only lead to a greater understanding of how these infections induce variable disease and immune responses in distinct species but also offer important insights into the evolution of mammalian immune systems.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

