Patch-clamp recordings and calcium imaging followed by single-cell PCR reveal the developmental profile of 13 genes in iPSC-derived human neurons
- PMID: 24157591
- PMCID: PMC3947234
- DOI: 10.1016/j.scr.2013.09.014
Patch-clamp recordings and calcium imaging followed by single-cell PCR reveal the developmental profile of 13 genes in iPSC-derived human neurons
Abstract
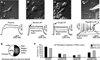
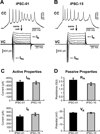
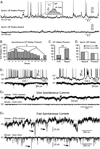
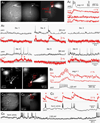
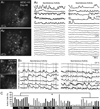
Molecular genetic studies are typically performed on homogenized biological samples, resulting in contamination from non-neuronal cells. To improve expression profiling of neurons we combined patch recordings with single-cell PCR. Two iPSC lines (healthy subject and 22q11.2 deletion) were differentiated into neurons. Patch electrode recordings were performed on 229 human cells from Day-13 to Day-88, followed by capture and single-cell PCR for 13 genes: ACTB, HPRT, vGLUT1, βTUBIII, COMT, DISC1, GAD1, PAX6, DTNBP1, ERBB4, FOXP1, FOXP2, and GIRK2. Neurons derived from both iPSC lines expressed βTUBIII, fired action potentials, and experienced spontaneous depolarizations (UP states) ~2 weeks before vGLUT1, GAD1 and GIRK2 appeared. Multisite calcium imaging revealed that these UP states were not synchronized among hESC-H9-derived neurons. The expression of FOXP1, FOXP2 and vGLUT1 was lost after 50 days in culture, in contrast to other continuously expressed genes. When gene expression was combined with electrophysiology, two subsets of genes were apparent; those irrelevant to spontaneous depolarizations (including vGLUT1, GIRK2, FOXP2 and DISC1) and those associated with spontaneous depolarizations (GAD1 and ERBB4). The results demonstrate that in the earliest stages of neuron development, it is useful to combine genetic analysis with physiological characterizations, on a cell-to-cell basis.
Keywords: AP; CC; EB; MEFs; NE; R(IN); V(R); VC; action potential; current clamp; embryoid bodies; input resistance.; mouse embryonic fibroblasts; neuroepithelial (rosettes); resting membrane potential; voltage clamp.
© 2013.
Figures



References
-
- Belinsky GS, Moore AR, Short SM, Rich MT, Antic SD. Physiological Properties of Neurons Derived from Human Embryonic Stem Cells using a dbcAMP-based Protocol. Stem Cells and Development. 2011;20(10):1733–1746. - PubMed
-
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

