Triggered Nanoparticles as Therapeutics
- PMID: 24159362
- PMCID: PMC3804356
- DOI: 10.1016/j.nantod.2013.07.004
Triggered Nanoparticles as Therapeutics
Abstract
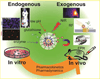
Drug delivery systems (DDSs) face several challenges including site-specific delivery, stability, and the programmed release of drugs. Engineered nanoparticle (NP) surfaces with responsive moieties can enhance the efficacy of DDSs for in vitro and in vivo systems. This triggering process can be achieved through both endogenous (biologically controlled release) and exogenous (external stimuli controlled release) activation. In this review, we will highlight recent examples of the use of triggered release strategies of engineered nanomaterials for in vitro and in vivo applications.
Keywords: Engineered nanoparticles; drug delivery system; in vitro; in vivo; triggered release.
Figures







References
-
- Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Nat. Rev. Cancer. 2012;12:39–50. - PubMed
-
- Allen TM, Cullis PR. Science. 2004;303:1818–1822. - PubMed
-
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Natl J. Cancer Inst. 2006;98:335–344. - PubMed
-
- Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv. Drug Deliv. Rev. 2008;60:1307–1315. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
