Neuroprotective Role of a Novel Copper Chelator against Aβ 42 Induced Neurotoxicity
- PMID: 24159420
- PMCID: PMC3789492
- DOI: 10.1155/2013/567128
Neuroprotective Role of a Novel Copper Chelator against Aβ 42 Induced Neurotoxicity
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease and associated with the extracellular deposits of amyloid- β peptide in hippocampus region. Metal ions like Cu, Fe and Zn are known to associate with the amyloid beta (A β ) at high concentration and interaction of these ions with soluble and aggregated forms of A β peptide help in development of AD. Here we showed Cu mediated neurotoxicity in the eye tissues of transgenic Drosophila expressing human amyloid β and its rescue through a novel Cu chelator. In this context, we have synthesised and characterized the compound L 2,6-Pyridinedicarboxylic acid, 2,6-bis[2-[(4-carboxyphenyl) methylene] hydrazide] by Mass spectra (MS) and Elemental analysis (EA). The Cu chelation potential of the compound L is tested in vivo in Drosophila. Oral administration of Copper to the transgenic larvae resulted in severe degeneration in eye tissues, which was rescued by the supplementation of compound L. The levels of anti-oxidant markers like SOD and MDA were measured in compound L treated flies and found a significant rescue (P < 0.001). Further rescue of the eye degeneration phenotypes as revealed by SEM affirm the role of copper in A β toxicity. Hence, use of compound L, an amidoamine derivative, could be a possible therapeutic measure for A β induced neurotoxicity.
Figures

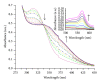
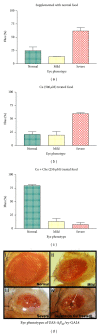



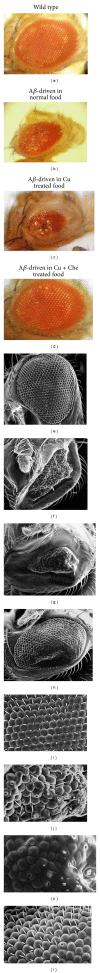
References
-
- Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Transactions. 2009;(7):1080–1094. - PubMed
-
- Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer's disease. Journal of Biological Inorganic Chemistry. 2009;15:61–76. - PubMed
-
- Crichton RR, Dexter DT, Ward RJ. Metal based neurodegenerative diseases—from molecular mechanisms to therapeutic strategies. Coordination Chemistry Reviews. 2008;252(10-11):1189–1199.
-
- Donnelly PS, Xiao Z, Wedd AG. Copper and Alzheimer’s disease. Current Opinion in Chemical Biology. 2007;11(2):128–133. - PubMed
-
- Hureau C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease—part 1: an overview. Coordination Chemistry Reviews. 2012;256(19-20):2164–2174.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

