Serological Correlate of Protection in Guinea Pigs for a Recombinant Protective Antigen Anthrax Vaccine Produced from Bacillus brevis
- PMID: 24159510
- PMCID: PMC3738701
- DOI: 10.1016/j.phrp.2012.07.006
Serological Correlate of Protection in Guinea Pigs for a Recombinant Protective Antigen Anthrax Vaccine Produced from Bacillus brevis
Abstract
Objective: Recombinant protective antigen (rPA) is the active pharmaceutical ingredient of a second generation anthrax vaccine undergoing clinical trials both in Korea and the USA. By using the rPA produced from Bacillus brevis pNU212 expression system, correlations of serological immune response to anthrax protection efficacy were analyzed in a guinea pig model.
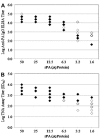
Methods: Serological responses of rPA anthrax vaccine were investigated in guinea pigs that were given single or two injections (interval of 4 weeks) of various amounts of rPA combined with aluminumhydroxide adjuvant. Guinea pigs were subsequently challenged by the intramuscular injection with 30 half-lethal doses (30LD50) of virulent Bacillus anthracis spores. Serumantibody titerswere determined by anti-PA IgGELISA and the ability of antibodies to neutralize the cytotoxicity of lethal toxin on J774A.1 cell was measured through the toxin neutralizing antibody (TNA) assay.
Results: To examine correlations between survival rate and antibody titers, correlation between neutralizing antibody titers and the extent of protection was determined. Toxin neutralization titers of at least 1176 were sufficient to confer protection against a dose of 30LD50 of virulent anthrax spores of the H9401 strain. Such consistency in the correlation was not observed from those antibody titers determined by ELISA.
Conclusion: Neutralizing-antibody titers can be used as a surrogate marker.
Keywords: Bacillus anthracis; Guinea pig; anthrax; serological correlate.
Figures

References
-
- Little SF, Ivins BE, Webster WM, et al. Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine. Vaccine. 2007 Apr;12(15):2771–7. - PubMed
-
- Edwards KA, Clancy HA, Baeumner AJ. Bacillus anthracis: toxicology, epidemiology and current rapid-detection methods. Anal Bioanal Chem. 2006 Jan;384(1):73–84. - PubMed
-
- Wang SH, Wen JK, Zhou YF, et al. Identification and characterization of Bacillus anthracis by multiplex PCR on DNA chip. Biosens Bioelectron. 2004 Nov;20(4):807–13. - PubMed
-
- Little SF, Ivins BE, Fellows PF, et al. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004 Jan;22(3-4):422–30. - PubMed
-
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–71. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

