An integrated strategy for efficient vector construction and multi-gene expression in Plasmodium falciparum
- PMID: 24160265
- PMCID: PMC3842810
- DOI: 10.1186/1475-2875-12-373
An integrated strategy for efficient vector construction and multi-gene expression in Plasmodium falciparum
Abstract
Background: The construction of plasmid vectors for transgene expression in the malaria parasite, Plasmodium falciparum, presents major technical hurdles. Traditional molecular cloning by restriction and ligation often yields deletions and re-arrangements when assembling low-complexity (A + T)-rich parasite DNA. Furthermore, the use of large 5'- and 3'- untranslated regions of DNA sequence (UTRs) to drive transgene transcription limits the number of expression cassettes that can be incorporated into plasmid vectors.
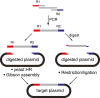
Methods: To address these challenges, two high fidelity cloning strategies, namely yeast homologous recombination and the Gibson assembly method, were evaluated for constructing P. falciparum vectors. Additionally, some general rules for reliably using the viral 2A-like peptide to express multiple proteins from a single expression cassette while preserving their proper trafficking to various subcellular compartments were assessed.
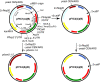
Results: Yeast homologous recombination and Gibson assembly were found to be effective strategies for successfully constructing P. falciparum plasmid vectors. Using these cloning methods, a validated family of expression vectors that provide a flexible starting point for user-specific applications was created. These vectors are also compatible with traditional cloning by restriction and ligation, and contain useful combinations of commonly used features for enhancing plasmid segregation and site-specific integration in P. falciparum. Additionally, application of a 2A-like peptide for the synthesis of multiple proteins from a single expression cassette, and some rules for combinatorially directing proteins to discrete subcellular compartments were established.
Conclusions: A set of freely available, sequence-verified and functionally validated parts that offer greater flexibility for constructing P. falciparum vectors having expanded expression capacity is provided.
Figures



Similar articles
-
Stable transgene expression in Plasmodium falciparum.Mol Biochem Parasitol. 1997 Dec 1;90(1):131-44. doi: 10.1016/s0166-6851(97)00143-6. Mol Biochem Parasitol. 1997. PMID: 9497038
-
Available methods for assembling expression cassettes for synthetic biology.Appl Microbiol Biotechnol. 2012 Mar;93(5):1853-63. doi: 10.1007/s00253-012-3920-8. Epub 2012 Feb 7. Appl Microbiol Biotechnol. 2012. PMID: 22311648 Review.
-
Yeast recombination-based cloning as an efficient way of constructing vectors for Zymoseptoria tritici.Fungal Genet Biol. 2015 Jun;79:76-83. doi: 10.1016/j.fgb.2015.03.017. Fungal Genet Biol. 2015. PMID: 26092792 Free PMC article.
-
Robust inducible Cre recombinase activity in the human malaria parasite Plasmodium falciparum enables efficient gene deletion within a single asexual erythrocytic growth cycle.Mol Microbiol. 2013 May;88(4):687-701. doi: 10.1111/mmi.12206. Epub 2013 Mar 26. Mol Microbiol. 2013. PMID: 23489321 Free PMC article.
-
Building mosaics of therapeutic plasmid gene vectors.Curr Gene Ther. 2011 Dec;11(6):466-78. doi: 10.2174/156652311798192798. Curr Gene Ther. 2011. PMID: 22023476 Review.
Cited by
-
Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum.Nat Methods. 2014 Sep;11(9):915-8. doi: 10.1038/nmeth.3063. Epub 2014 Aug 10. Nat Methods. 2014. PMID: 25108687 Free PMC article.
-
An integrated platform for genome engineering and gene expression perturbation in Plasmodium falciparum.Sci Rep. 2021 Jan 11;11(1):342. doi: 10.1038/s41598-020-77644-4. Sci Rep. 2021. PMID: 33431920 Free PMC article.
-
Genetic Indicators for Calcium Signaling Studies in Toxoplasma gondii.Methods Mol Biol. 2020;2071:187-207. doi: 10.1007/978-1-4939-9857-9_11. Methods Mol Biol. 2020. PMID: 31758454 Free PMC article.
-
Phosphatidylinositol 3-phosphate and Hsp70 protect Plasmodium falciparum from heat-induced cell death.Elife. 2020 Sep 25;9:e56773. doi: 10.7554/eLife.56773. Elife. 2020. PMID: 32975513 Free PMC article.
-
A mutagenesis screen for essential plastid biogenesis genes in human malaria parasites.PLoS Biol. 2019 Feb 6;17(2):e3000136. doi: 10.1371/journal.pbio.3000136. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30726238 Free PMC article.
References
-
- WHO. World Malaria Report: 2012. Geneva: World Health Organization; 2012.
-
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan M-S, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DMA, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. - DOI - PMC - PubMed
-
- Bowman S, Lawson D, Basham D, Brown D, Chillingworth T, Churcher CM, Craig A, Davies RM, Devlin K, Feltwell T, Gentles S, Gwilliam R, Hamlin N, Harris D, Holroyd S, Hornsby T, Horrocks P, Jagels K, Jassal B, Kyes S, McLean J, Moule S, Mungall K, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutter S, Skelton J, Squares R, Squares S, Sulston JE, Whitehead S, Woodward JR, Newbold C, Barrell BG. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature. 1999;400:532–538. doi: 10.1038/22964. - DOI - PubMed
-
- Gardner MJ, Tettelin H, Carucci DJ, Cummings LM, Aravind L, Koonin EV, Shallom S, Mason T, Yu K, Fujii C, Pederson J, Shen K, Jing J, Aston C, Lai Z, Schwartz DC, Pertea M, Salzberg S, Zhou L, Sutton GG, Clayton R, White O, Smith HO, Fraser CM, Adams MD, Venter JC, Hoffman SL. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science. 1998;282:1126–1132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

