A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse
- PMID: 24160371
- PMCID: PMC3819659
- DOI: 10.1186/1745-6215-14-353
A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse
Abstract
Background: Current Medical Research Council (MRC) guidance on complex interventions advocates pilot trials and feasibility studies as part of a phased approach to the development, testing, and evaluation of healthcare interventions. In this paper we discuss the results of a recent feasibility study and pilot trial for a randomized controlled trial (RCT) of pelvic floor muscle training for prolapse (ClinicalTrials.gov: NCT01136889). The ways in which researchers decide to respond to the results of feasibility work may have significant repercussions for both the nature and degree of tension between internal and external validity in a definitive trial.
Methods: We used methodological issues to classify and analyze the problems that arose in the feasibility study. Four centers participated with the aim of randomizing 50 women. Women were eligible if they had prolapse of any type, of stage I to IV, and had a pessary successfully fitted. Postal questionnaires were administered at baseline, 6 months, and 7 months post-randomization. After identifying problems arising within the pilot study we then sought to locate potential solutions that might minimize the trade-off between a subsequent explanatory versus pragmatic trial.
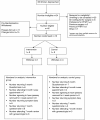
Results: The feasibility study pointed to significant potential problems in relation to participant recruitment, features of the intervention, acceptability of the intervention to participants, and outcome measurement. Finding minimal evidence to support our decision-making regarding the transition from feasibility work to a trial, we developed a systematic process (A process for Decision-making after Pilot and feasibility Trials (ADePT)) which we subsequently used as a guide. The process sought to: 1) encourage the systematic identification and appraisal of problems and potential solutions; 2) improve the transparency of decision-making processes; and 3) reveal the tensions that exist between pragmatic and explanatory choices.
Conclusions: We have developed a process that may aid researchers in their attempt to identify the most appropriate solutions to problems identified within future pilot and feasibility RCTs. The process includes three key steps: a decision about the type of problem, the identification of all solutions (whether addressed within the intervention, trial design or clinical context), and a systematic appraisal of these solutions.
Figures
References
-
- Medical Research Council (MRC) Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
-
- Øvretveit J. Evaluating complex social interventions. Eurohealth. 2004;10(2):37–40.
-
- Cooksey D. A Review of UK Health Research Funding. London: The Stationery Office; 2006.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical