Stromal cell-derived factor-1 promoted angiogenesis and inflammatory cell infiltration in aneurysm walls
- PMID: 24160472
- PMCID: PMC3877706
- DOI: 10.3171/2013.9.JNS122074
Stromal cell-derived factor-1 promoted angiogenesis and inflammatory cell infiltration in aneurysm walls
Abstract
Object: A small percentage of cerebral aneurysms rupture, but when they do, the effects are devastating. Current management of unruptured aneurysms consists of surgery, endovascular treatment, or watchful waiting. If the biology of how aneurysms grow and rupture were better known, a novel drug could be developed to prevent unruptured aneurysms from rupturing. Ruptured cerebral aneurysms are characterized by inflammation-mediated wall remodeling. The authors studied the role of stromal cell-derived factor-1 (SDF-1) in inflammation-mediated wall remodeling in cerebral aneurysms.
Methods: Human aneurysms, murine carotid artery aneurysms, and murine intracranial aneurysms were studied using immunohistochemistry. Flow cytometry analysis was performed on blood from mice developing carotid or intracranial aneurysms. The effect of SDF-1 on endothelial cells and macrophages was studied by chemotaxis cell migration assay and capillary tube formation assay. Anti-SDF-1 blocking antibody was given to mice and compared with control (vehicle)-administered mice for its effects on the walls of carotid aneurysms and the development of intracranial aneurysms.
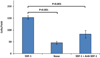
Results: Human aneurysms, murine carotid aneurysms, and murine intracranial aneurysms all expressed SDF-1, and mice with developing carotid or intracranial aneurysms had increased progenitor cells expressing CXCR4, the receptor for SDF-1 (p < 0.01 and p < 0.001, respectively). Human aneurysms and murine carotid aneurysms had endothelial cells, macrophages, and capillaries in the walls of the aneurysms, and the presence of capillaries in the walls of human aneurysms was associated with the presence of macrophages (p = 0.01). Stromal cell-derived factor-1 promoted endothelial cell and macrophage migration (p < 0.01 for each), and promoted capillary tube formation (p < 0.001). When mice were given anti-SDF-1 blocking antibody, there was a significant reduction in endothelial cells (p < 0.05), capillaries (p < 0.05), and cell proliferation (p < 0.05) in the aneurysm wall. Mice given anti-SDF-1 blocking antibody developed significantly fewer intracranial aneurysms (33% vs 89% in mice given control immunoglobulin G, respectively; p < 0.05).
Conclusions: These data suggest SDF-1 is associated with angiogenesis and inflammatory cell migration and proliferation in the walls of aneurysms, and may have a role in the development of intracranial aneurysms.
Conflict of interest statement
CONFLICTS OF INTEREST/DISCLOSURES
None
Figures


















Comment in
-
Aneurysm wall inflammation.J Neurosurg. 2014 Jan;120(1):70-2. doi: 10.3171/2013.5.JNS13824. Epub 2013 Oct 25. J Neurosurg. 2014. PMID: 24160480 No abstract available.
References
-
- Atkinson JL, Okazaki H, Sundt TM, Jr., Nichols DA, Rufenacht DA. Intracranial cerebrovascular vasa vasorum associated with atherosclerosis and large thick-walled aneurysms. Surg Neurol. 1991;36:365–369. - PubMed
-
- Bavinzski G, Talazoglu V, Killer M, Richling B, Gruber A, Gross CE, Plenk H., Jr. Gross and microscopic histopathologic findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–293. - PubMed
-
- Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT) Stroke. 2007;38:1538–1544. - PubMed
-
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. - PubMed
-
- Chen T, Bai H, Shao Y, Arzigian M, Janzen V, Attar E, Xie Y, Scadden DT, Wang ZZ. Stromal cell-derived factor-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

