Regenerative medicine approach to reconstruction of the equine upper airway
- PMID: 24160675
- PMCID: PMC3993024
- DOI: 10.1089/ten.TEA.2013.0217
Regenerative medicine approach to reconstruction of the equine upper airway
Abstract
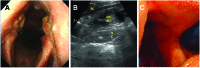
Airway obstruction is a common cause of poor performance in horses. Structural abnormalities (insufficient length, rigidity) can be a cause for the obstruction. Currently, there are a few effective clinical options for reconstruction of the equine larynx. A regenerative medicine approach to reconstruction may provide the capability to stabilize laryngeal structures and to encourage restoration of site-appropriate, functional, and host-derived tissue. The purpose of this study was the histopathological evaluation of (1) decellularization of equine (horse) laryngeal cartilages (epiglottis and arytenoids); (2) the host response to decellularized laryngeal cartilages implanted subcutaneously in a donkey model as a test of biocompatibility; and (3) the use of decellularized laryngeal cartilages in a clinically relevant pilot study in the horse larynx. Equine laryngeal cartilages were found to be sufficiently decellularized and were subsequently implanted subcutaneously in donkeys to test biocompatibility. After 4 weeks, the implanted cartilage was harvested. In the subcutaneous model, the samples did not elicit a rejection or foreign body type reaction and were judged suitable for implantation in a clinically relevant equine model. Implants were placed in the upper airway (arytenoids and epiglottis) of one horse. At 4 weeks, the implants were observed to remodel rapidly and were replaced by dense connective tissue with signs of new hyaline cartilage formation in the arytenoids and by connective tissue containing glandular structures and an epithelial covering in the epiglottis. The results of the present study demonstrate the feasibility of a scaffold-based regenerative medicine approach to reconstruction of the equine upper airway; however, further studies investigating long-term integration, formation of new cartilage, and mechanical properties are needed.
Figures





References
-
- Butler P.J., Woakes A.J., Smale K., Roberts C.A., Hillidge C.J., Snow D.H., and Marlin D.J.Respiratory and cardiovascular adjustments during exercise of increasing intensity and during recovery in thoroughbred racehorses. J Exp Biol 179,159, 1993 - PubMed
-
- Tetens J., Derksen F.J., Stick J.A., Lloyd J.W., and Robinson N.E.Efficacy of prosthetic laryngoplasty with and without bilateral ventriculocordectomy as treatments for laryngeal hemiplegia in horses. Am J Vet Res 57,1668, 1996 - PubMed
-
- Holcombe S.J., and Ducharme N.G.Upper airway function of normal horses during exercise. In: Hinchcliff K.W., Kaneps A.J., and Geor R.J., eds. Equine Sports Medicine and Surgery. Basic and Clinical Sciences of the Equine Athlete. Edinburgh: W.B. Saunders, p. 541, 2004
-
- Derksen F.J., Robinson N.E., and Holcombe S.J.The upper airway as a conduit for air flow. In: Auer J.A., and Stick J.A., eds. Equine Surgery. Philadelphia: W.B. Saunders, p. 307, 1999
-
- Ducharme N.G., Hackett R.P., Ainsworth D.M., Erb H.N., and Shannon K.J.Repeatability and normal values for measurement of pharyngeal and tracheal pressures in exercising horses. Am J Vet Res 55,368, 1994 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

