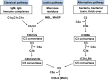
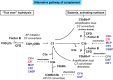
Overview of complement activation and regulation
- PMID: 24161035
- PMCID: PMC3820029
- DOI: 10.1016/j.semnephrol.2013.08.001
Overview of complement activation and regulation
Abstract
Complement is an important component of the innate immune system that is crucial for defense from microbial infections and for clearance of immune complexes and injured cells. In normal conditions complement is tightly controlled by a number of fluid-phase and cell surface proteins to avoid injury to autologous tissues. When complement is hyperactivated, as occurs in autoimmune diseases or in subjects with dysfunctional regulatory proteins, it drives a severe inflammatory response in numerous organs. The kidney appears to be particularly vulnerable to complement-mediated inflammatory injury. Injury may derive from deposition of circulating active complement fragments in glomeruli, but complement locally produced and activated in the kidney also may have a role. Many kidney disorders have been linked to abnormal complement activation, including immune-complex-mediated glomerulonephritis and rare genetic kidney diseases, but also tubulointerstitial injury associated with progressive proteinuric diseases or ischemia-reperfusion.
Keywords: Complement; adaptive immunity; complement regulators; innate immunity; kidney; kidney diseases.
© 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Walport M.J. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. - PubMed
-
- Walport M.J. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. - PubMed
-
- Botto M., Kirschfink M., Macor P., Pickering M.C., Wurzner R., Tedesco F. Complement in human diseases: lessons from complement deficiencies. Mol Immunol. 2009;46:2774–2783. - PubMed
-
- Zipfel P.F., Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

