Complement in ANCA-associated vasculitis
- PMID: 24161040
- PMCID: PMC4083854
- DOI: 10.1016/j.semnephrol.2013.08.006
Complement in ANCA-associated vasculitis
Abstract
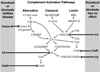
Antineutrophil cytoplasmic autoantibodies (ANCA) are the likely cause for necrotizing small-vessel vasculitis and crescentic glomerulonephritis. Unlike other forms of crescentic glomerulonephritis induced by immune complexes or anti-glomerular basement membrane antibodies that have conspicuous vessel wall immunoglobulin and complement, there is a paucity, although usually not an absence, of vessel wall immunoglobulin and complement in ANCA-associated glomerulonephritis. Despite this comparatively lower level and more localized distribution of vessel wall complement, experimental and clinical observations strongly incriminate alternative complement pathway activation as critically important in the pathogenesis of ANCA disease. Experimental data in animal models and in vitro experiments has shown that primed neutrophils are activated by ANCA, which generates C5a, which engages C5a receptors on neutrophils. This attracts and in turn primes more neutrophils for activation by ANCA. In patients with ANCA disease, plasma levels of C3a, C5a, soluble C5b-9, and Bb have been reported to be higher in active disease than in remission, whereas no difference was reported in plasma C4d in active versus ANCA disease remission. Thus, experimental and clinical data support the hypothesis that ANCA-induced neutrophil activation activates the alternative complement pathway and generates C5a. C5a not only recruits additional neutrophils through chemotaxis but also primes neutrophils for activation by ANCA. This creates a self-fueling inflammatory amplification loop that results in the extremely destructive necrotizing vascular injury.
Keywords: ANCA; antineutrophil cytoplasmic autoantibodies; crescentic glomerulonephritis; vasculitis.
© 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Falk RJ, Jennette JC. ANCA Disease: Where Is This Field Going? J Am Soc Nephrol. 2010;21:745–752. - PubMed
-
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CGM, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DGI, Specks U, Stone JH, Takahashi K, Watts RA. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. - PubMed
-
- Jennette JC, Falk RJ. The role of pathology in the diagnosis of systemic vasculitis. Clin Exp Rheumatol. 2007;25(1 Suppl 44):52–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

