Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial
- PMID: 24161233
- PMCID: PMC3895323
- DOI: 10.1016/S1473-3099(13)70295-0
Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial
Erratum in
- Lancet Infect Dis. 2014 Jan;14(1):11
Abstract
Background: Intensive care units (ICUs) are high-risk areas for transmission of antimicrobial-resistant bacteria, but no controlled study has tested the effect of rapid screening and isolation of carriers on transmission in settings with best-standard precautions. We assessed interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in European ICUs.
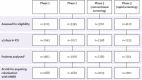
Methods: We did this study in three phases at 13 ICUs. After a 6 month baseline period (phase 1), we did an interrupted time series study of universal chlorhexidine body-washing combined with hand hygiene improvement for 6 months (phase 2), followed by a 12-15 month cluster randomised trial (phase 3). ICUs were randomly assigned by computer generated randomisation schedule to either conventional screening (chromogenic screening for meticillin-resistant Staphylococcus aureus [MRSA] and vancomycin-resistant enterococci [VRE]) or rapid screening (PCR testing for MRSA and VRE and chromogenic screening for highly resistant Enterobacteriaceae [HRE]); with contact precautions for identified carriers. The primary outcome was acquisition of resistant bacteria per 100 patient-days at risk, for which we calculated step changes and changes in trends after the introduction of each intervention. We assessed acquisition by microbiological surveillance and analysed it with a multilevel Poisson segmented regression model. We compared screening groups with a likelihood ratio test that combined step changes and changes to trend. This study is registered with ClinicalTrials.gov, number NCT00976638.
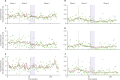
Findings: Seven ICUs were assigned to rapid screening and six to conventional screening. Mean hand hygiene compliance improved from 52% in phase 1 to 69% in phase 2, and 77% in phase 3. Median proportions of patients receiving chlorhexidine body-washing increased from 0% to 100% at the start of phase 2. For trends in acquisition of antimicrobial-resistant bacteria, weekly incidence rate ratio (IRR) was 0·976 (0·954-0·999) for phase 2 and 1·015 (0·998-1·032) for phase 3. For step changes, weekly IRR was 0·955 (0·676-1·348) for phase 2 and 0·634 (0·349-1·153) for phase 3. The decrease in trend in phase 2 was largely caused by changes in acquisition of MRSA (weekly IRR 0·925, 95% CI 0·890-0·962). Acquisition was lower in the conventional screening group than in the rapid screening group, but did not differ significantly (p=0·06).
Interpretation: Improved hand hygiene plus unit-wide chlorhexidine body-washing reduced acquisition of antimicrobial-resistant bacteria, particularly MRSA. In the context of a sustained high level of compliance to hand hygiene and chlorhexidine bathings, screening and isolation of carriers do not reduce acquisition rates of multidrug-resistant bacteria, whether or not screening is done with rapid testing or conventional testing.
Funding: European Commission.
Copyright © 2014 Derde et al. Open Access article distributed under the terms of CC BY-NC-SA. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Antimicrobial resistance in intensive care units.Lancet Infect Dis. 2014 Jan;14(1):3-5. doi: 10.1016/S1473-3099(13)70305-0. Epub 2013 Oct 23. Lancet Infect Dis. 2014. PMID: 24161232 No abstract available.
-
Care bundles in intensive care units.Lancet Infect Dis. 2014 May;14(5):371-2. doi: 10.1016/S1473-3099(14)70731-5. Lancet Infect Dis. 2014. PMID: 24758993 No abstract available.
-
Care bundles in intensive care units - authors' reply.Lancet Infect Dis. 2014 May;14(5):372. doi: 10.1016/S1473-3099(14)70740-6. Lancet Infect Dis. 2014. PMID: 24758996 No abstract available.
References
-
- Goldmann DA, Weinstein RA, Wenzel RP. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA. 1996;275:234–240. - PubMed
-
- WHO . WHO guidelines on hand hygiene in health care. World Health Organization; Geneva: 2009.
-
- Batra R, Cooper BS, Whiteley C, Patel AK, Wyncoll D, Edgeworth JD. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2010;50:210–217. - PubMed
-
- Climo MW, Sepkowitz KA, Zuccotti G. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–1865. - PubMed
-
- Gould IM, MacKenzie FM, MacLennan G, Pacitti D, Watson EJ, Noble DW. Topical antimicrobials in combination with admission screening and barrier precautions to control endemic methicillin-resistant Staphylococcus aureus in an intensive care unit. Int J Antimicrob Ag. 2007;29:536–543. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

