Poxviruses and the evolution of host range and virulence
- PMID: 24161410
- PMCID: PMC3945082
- DOI: 10.1016/j.meegid.2013.10.014
Poxviruses and the evolution of host range and virulence
Abstract
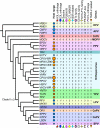
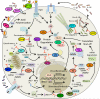
Poxviruses as a group can infect a large number of animals. However, at the level of individual viruses, even closely related poxviruses display highly diverse host ranges and virulence. For example, variola virus, the causative agent of smallpox, is human-specific and highly virulent only to humans, whereas related cowpox viruses naturally infect a broad spectrum of animals and only cause relatively mild disease in humans. The successful replication of poxviruses depends on their effective manipulation of the host antiviral responses, at the cellular-, tissue- and species-specific levels, which constitutes a molecular basis for differences in poxvirus host range and virulence. A number of poxvirus genes have been identified that possess host range function in experimental settings, and many of these host range genes target specific antiviral host pathways. Herein, we review the biology of poxviruses with a focus on host range, zoonotic infections, virulence, genomics and host range genes as well as the current knowledge about the function of poxvirus host range factors and how their interaction with the host innate immune system contributes to poxvirus host range and virulence. We further discuss the evolution of host range and virulence in poxviruses as well as host switches and potential poxvirus threats for human and animal health.
Keywords: Antiviral response; Host range; Host response; Host–pathogen interactions; Poxviruses; Virus evolution.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

References
-
- Abrahao JS, Guedes MI, Trindade GS, Fonseca FG, Campos RK, Mota BF, Lobato ZI, Silva-Fernandes AT, Rodrigues GO, Lima LS, Ferreira PC, Bonjardim CA, Kroon EG. One more piece in the VACV ecological puzzle: could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PloS one. 2009;4:e7428. - PMC - PubMed
-
- Abubakr MI, Abu-Elzein EM, Housawi FM, Abdelrahman AO, Fadlallah ME, Nayel MN, Adam AS, Moss S, Forrester NL, Coloyan E, Gameel A, Al- Afaleq AI, Gould EA. Pseudocowpox virus: the etiological agent of contagious ecthyma (Auzdyk) in camels (Camelus dromedarius) in the Arabian peninsula. Vector Borne Zoonotic Dis. 2007;7:257–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

