Induction of a chloracne phenotype in an epidermal equivalent model by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is dependent on aryl hydrocarbon receptor activation and is not reproduced by aryl hydrocarbon receptor knock down
- PMID: 24161567
- PMCID: PMC3885976
- DOI: 10.1016/j.jdermsci.2013.09.001
Induction of a chloracne phenotype in an epidermal equivalent model by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is dependent on aryl hydrocarbon receptor activation and is not reproduced by aryl hydrocarbon receptor knock down
Abstract
Background: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a potent activator of the aryl hydrocarbon receptor (AhR) and causes chloracne in humans. The pathogenesis and role of AhR in chloracne remains incompletely understood.
Objective: To elucidate the mechanisms contributing to the development of the chloracne-like phenotype in a human epidermal equivalent model and identify potential biomarkers.
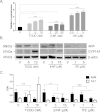
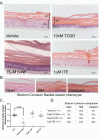
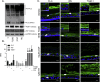
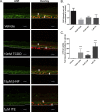
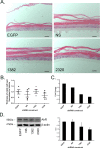
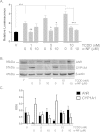
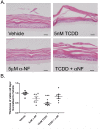
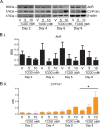
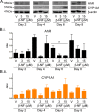
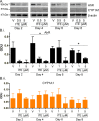
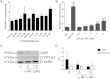
Methods: Using primary normal human epidermal keratinocytes (NHEK), we studied AhR activation by XRE-luciferase, AhR degradation and CYP1A1 induction. We treated epidermal equivalents with high affinity TCDD or two non-chloracnegens: β-naphthoflavone (β-NF) and 2-(1'H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE). Using Western blotting and immunochemistry for filaggrin (FLG), involucrin (INV) and transglutaminase-1 (TGM-1), we compared the effects of the ligands on keratinocyte differentiation and development of the chloracne-like phenotype by H&E.
Results: In NHEKs, activation of an XRE-luciferase and CYP1A1 protein induction correlated with ligand binding affinity: TCDD>β-NF>ITE. AhR degradation was induced by all ligands. In epidermal equivalents, TCDD induced a chloracne-like phenotype, whereas β-NF or ITE did not. All three ligands induced involucrin and TGM-1 protein expression in epidermal equivalents whereas FLG protein expression decreased following treatment with TCDD and β-NF. Inhibition of AhR by α-NF blocked TCDD-induced AhR activation in NHEKs and blocked phenotypic changes in epidermal equivalents; however, AhR knock down did not reproduce the phenotype.
Conclusion: Ligand-induced CYP1A1 and AhR degradation did not correlate with their chloracnegenic potential, indicating that neither CYP1A1 nor AhR are suitable biomarkers. Mechanistic studies showed that the TCDD-induced chloracne-like phenotype depends on AhR activation whereas AhR knock down did not appear sufficient to induce the phenotype.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester; AHRR; AhR; Aryl hydrocarbon receptor; CYP1A1; Epidermal equivalent; ITE; Keratinocyte; TCDD; TGM-1; XRE; aryl hydrocarbon receptor; aryl hydrocarbon repressor protein; cytochrome P450 1A1; transglutaminase-1; xenobiotic response element; α-NF; α-naphthoflavone; β-NF; β-Naphthoflavone; β-naphthoflavone.
Copyright © 2013 Japanese Society for Investigative Dermatology. Published by Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo.Arch Biochem Biophys. 2006 Jun 1;450(1):67-77. doi: 10.1016/j.abb.2006.02.008. Epub 2006 Mar 3. Arch Biochem Biophys. 2006. PMID: 16545771
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation.Toxicol Sci. 2011 Nov;124(1):128-37. doi: 10.1093/toxsci/kfr205. Epub 2011 Aug 11. Toxicol Sci. 2011. PMID: 21835898 Free PMC article.
-
Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines.Toxicol Appl Pharmacol. 2014 Feb 1;274(3):408-16. doi: 10.1016/j.taap.2013.12.002. Epub 2013 Dec 16. Toxicol Appl Pharmacol. 2014. PMID: 24355420 Free PMC article.
-
Functional role of AhR in the expression of toxic effects by TCDD.Biochim Biophys Acta. 2003 Feb 17;1619(3):263-8. doi: 10.1016/s0304-4165(02)00485-3. Biochim Biophys Acta. 2003. PMID: 12573486 Review.
-
Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor.Arch Biochem Biophys. 2005 Jan 15;433(2):379-86. doi: 10.1016/j.abb.2004.09.031. Arch Biochem Biophys. 2005. PMID: 15581594 Review.
Cited by
-
Reoxygenation induces reactive oxygen species production and ferroptosis in renal tubular epithelial cells by activating aryl hydrocarbon receptor.Mol Med Rep. 2021 Jan;23(1):41. doi: 10.3892/mmr.2020.11679. Epub 2020 Nov 12. Mol Med Rep. 2021. PMID: 33179104 Free PMC article.
-
2,3,7,8‑Tetrachlorodibenzo‑p‑dioxin suppresses the growth of human liver cancer HepG2 cells in vitro: Involvement of cell signaling factors.Int J Oncol. 2018 Oct;53(4):1657-1666. doi: 10.3892/ijo.2018.4507. Epub 2018 Jul 27. Int J Oncol. 2018. PMID: 30066859 Free PMC article.
-
Aryl Hydrocarbon Receptor and Dioxin-Related Health Hazards-Lessons from Yusho.Int J Mol Sci. 2021 Jan 12;22(2):708. doi: 10.3390/ijms22020708. Int J Mol Sci. 2021. PMID: 33445793 Free PMC article. Review.
-
Particulate Matter-Induced Aryl Hydrocarbon Receptor Regulates Autophagy in Keratinocytes.Biomol Ther (Seoul). 2019 Nov 1;27(6):570-576. doi: 10.4062/biomolther.2019.025. Biomol Ther (Seoul). 2019. PMID: 30971064 Free PMC article.
-
Endocrine-disrupting chemicals and skin manifestations.Rev Endocr Metab Disord. 2016 Sep;17(3):449-457. doi: 10.1007/s11154-016-9371-2. Rev Endocr Metab Disord. 2016. PMID: 27363826 Review.
References
-
- Abbott B.D., Schmid J.E., Brown J.G., Wood C.R., White R.D., Buckalew A.R. RT-PCR quantification of AHR, ARNT, GR, and CYP1A1 mRNA in craniofacial tissues of embryonic mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and hydrocortisone. Toxicol Sci. 1999;47:76–85. - PubMed
-
- Fernandez-Salguero P.M., Ward J.M., Sundberg J.P., Gonzalez F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. - PubMed
-
- Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T.N., Nakao K. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. - PubMed
-
- Caputo R., Monti M., Ermacora E., Carminati G., Gelmetti C., Gianotti R. Cutaneous manifestations of tetrachlorodibenzo-p-dioxin in children and adolescents. Follow-up 10 years after the Seveso, Italy, accident. J Am Acad Dermatol. 1988;19:812–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

