TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling
- PMID: 24161934
- PMCID: PMC4006312
- DOI: 10.1038/ncb2861
TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling
Abstract
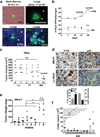
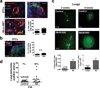
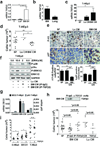
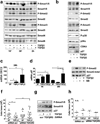
In patients, non-proliferative disseminated tumour cells (DTCs) can persist in the bone marrow (BM) while other organs (such as lung) present growing metastasis. This suggested that the BM might be a metastasis 'restrictive soil' by encoding dormancy-inducing cues in DTCs. Here we show in a head and neck squamous cell carcinoma (HNSCC) model that strong and specific transforming growth factor-β2 (TGF-β2) signalling in the BM activates the MAPK p38α/β, inducing an (ERK/p38)(low) signalling ratio. This results in induction of DEC2/SHARP1 and p27, downregulation of cyclin-dependent kinase 4 (CDK4) and dormancy of malignant DTCs. TGF-β2-induced dormancy required TGF-β receptor-I (TGF-β-RI), TGF-β-RIII and SMAD1/5 activation to induce p27. In lungs, a metastasis 'permissive soil' with low TGF-β2 levels, DTC dormancy was short-lived and followed by metastatic growth. Importantly, systemic inhibition of TGF-β-RI or p38α/β activities awakened dormant DTCs, fuelling multi-organ metastasis. Our work reveals a 'seed and soil' mechanism where TGF-β2 and TGF-β-RIII signalling through p38α/β regulates DTC dormancy and defines restrictive (BM) and permissive (lung) microenvironments for HNSCC metastasis.
Figures



References
-
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
-
- Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21:42–49. - PubMed
-
- Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202–207. - PubMed
-
- Zhang Y, Ma B, Fan Q. Mechanisms of breast cancer bone metastasis. Cancer Letters. 2010;292:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

