Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations
- PMID: 24162058
- PMCID: PMC3810609
- DOI: 10.1097/HP.0b013e31829cf221
Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations
Abstract
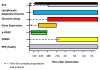
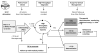
Following a mass-casualty nuclear disaster, effective medical triage has the potential to save tens of thousands of lives. In order to best use the available scarce resources, there is an urgent need for biodosimetry tools to determine an individual's radiation dose. Initial triage for radiation exposure will include location during the incident, symptoms, and physical examination. Stepwise triage will include point of care assessment of less than or greater than 2 Gy, followed by secondary assessment, possibly with high throughput screening, to further define an individual's dose. Given the multisystem nature of radiation injury, it is unlikely that any single biodosimetry assay can be used as a standalone tool to meet the surge in capacity with the timeliness and accuracy needed. As part of the national preparedness and planning for a nuclear or radiological incident, the authors reviewed the primary literature to determine the capabilities and limitations of a number of biodosimetry assays currently available or under development for use in the initial and secondary triage of patients. Understanding the requirements from a response standpoint and the capability and logistics for the various assays will help inform future biodosimetry technology development and acquisition. Factors considered include: type of sample required, dose detection limit, time interval when the assay is feasible biologically, time for sample preparation and analysis, ease of use, logistical requirements, potential throughput, point-of-care capability, and the ability to support patient diagnosis and treatment within a therapeutically relevant time point.
Figures


References
-
- Adalja AA, Watson M, Wollner S, Toner E. A possible approach to large-scale laboratory testing for acute radiation sickness after a nuclear detonation. Biosecur Bioterror. 2011;9:345–350. - PubMed
-
- Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V, Darroudi F, Fattibene P, Gruel G, Guclu I, Horn S, Jaworska A, Kulka U, Lindholm C, Lloyd D, Longo A, Marrale M, Monteiro Gil O, Oestreicher U, Pajic J, Rakic B, Romm H, Trompier F, Veronese I, Voisin P, Vral A, Whitehouse CA, Wieser A, Woda C, Wojcik A, Rothkamm K. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat Prot Dosim. 2011;147:573–592. - PubMed
-
- Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ., Jr. Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res. 2001;156:657–661. - PubMed
-
- Amundson SA, Fornace AJ., Jr. Gene expression profiles for monitoring radiation exposure. Radiat Prot Dosim. 2001;97:11–16. - PubMed
-
- Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol. 2009;85:369–376. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

