Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC
- PMID: 24162108
- PMCID: PMC4134870
- DOI: 10.1007/s00262-013-1493-8
Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC
Abstract
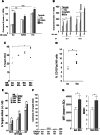
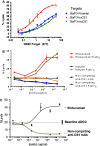
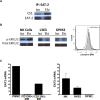
Elotuzumab is a monoclonal antibody in development for multiple myeloma (MM) that targets CS1, a cell surface glycoprotein expressed on MM cells. In preclinical models, elotuzumab exerts anti-MM efficacy via natural killer (NK)-cell-mediated antibody-dependent cellular cytotoxicity (ADCC). CS1 is also expressed at lower levels on NK cells where it acts as an activating receptor. We hypothesized that elotuzumab may have additional mechanisms of action via ligation of CS1 on NK cells that complement ADCC activity. Herein, we show that elotuzumab appears to induce activation of NK cells by binding to NK cell CS1 which promotes cytotoxicity against CS1(+) MM cells but not against autologous CS1(+) NK cells. Elotuzumab may also promote CS1-CS1 interactions between NK cells and CS1(+) target cells to enhance cytotoxicity in a manner independent of ADCC. NK cell activation appears dependent on differential expression of the signaling intermediary EAT-2 which is present in NK cells but absent in primary, human MM cells. Taken together, these data suggest elotuzumab may enhance NK cell function directly and confer anti-MM efficacy by means beyond ADCC alone.
Conflict of interest statement
Don M Benson has served in an advisory capacity to and received research funding from Bristol Myers Squibb; Gary C Starling is a former employee of Abbott Laboratories; Hakju Kwon and Audie Rice are employees of Abbvie Biotherapeutics Inc. No other authors have conflicts of interest to disclose.
Figures

Similar articles
-
Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways.Cancer Immunol Immunother. 2015 Jan;64(1):61-73. doi: 10.1007/s00262-014-1610-3. Epub 2014 Oct 7. Cancer Immunol Immunother. 2015. PMID: 25287778 Free PMC article.
-
Enhanced SLAMF7 Homotypic Interactions by Elotuzumab Improves NK Cell Killing of Multiple Myeloma.Cancer Immunol Res. 2019 Oct;7(10):1633-1646. doi: 10.1158/2326-6066.CIR-18-0579. Epub 2019 Aug 20. Cancer Immunol Res. 2019. PMID: 31431433 Free PMC article.
-
Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review.
-
CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma.Clin Cancer Res. 2008 May 1;14(9):2775-84. doi: 10.1158/1078-0432.CCR-07-4246. Clin Cancer Res. 2008. PMID: 18451245 Free PMC article.
-
CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma.Crit Rev Oncol Hematol. 2013 Oct;88(1):168-77. doi: 10.1016/j.critrevonc.2013.04.003. Epub 2013 Jun 2. Crit Rev Oncol Hematol. 2013. PMID: 23731618 Review.
Cited by
-
Hypergravity-induced changes in actin response of breast cancer cells to natural killer cells.Sci Rep. 2021 Mar 31;11(1):7267. doi: 10.1038/s41598-021-86799-7. Sci Rep. 2021. PMID: 33790394 Free PMC article.
-
Immune System Alterations in Multiple Myeloma: Molecular Mechanisms and Therapeutic Strategies to Reverse Immunosuppression.Cancers (Basel). 2021 Mar 17;13(6):1353. doi: 10.3390/cancers13061353. Cancers (Basel). 2021. PMID: 33802806 Free PMC article. Review.
-
Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions.Pharmaceuticals (Basel). 2024 Jul 6;17(7):901. doi: 10.3390/ph17070901. Pharmaceuticals (Basel). 2024. PMID: 39065751 Free PMC article. Review.
-
Monoclonal Antibody: A New Treatment Strategy against Multiple Myeloma.Antibodies (Basel). 2017 Nov 14;6(4):18. doi: 10.3390/antib6040018. Antibodies (Basel). 2017. PMID: 31548533 Free PMC article. Review.
-
Immunotherapy: A Novel Era of Promising Treatments for Multiple Myeloma.Int J Mol Sci. 2018 Nov 15;19(11):3613. doi: 10.3390/ijms19113613. Int J Mol Sci. 2018. PMID: 30445802 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

