System identification of the nonlinear dynamics in the thalamocortical circuit in response to patterned thalamic microstimulation in vivo
- PMID: 24162186
- PMCID: PMC4064456
- DOI: 10.1088/1741-2560/10/6/066011
System identification of the nonlinear dynamics in the thalamocortical circuit in response to patterned thalamic microstimulation in vivo
Abstract
Objective: Nonlinear system identification approaches were used to develop a dynamical model of the network level response to patterns of microstimulation in vivo.
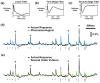
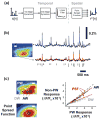
Approach: The thalamocortical circuit of the rodent vibrissa pathway was the model system, with voltage sensitive dye imaging capturing the cortical response to patterns of stimulation delivered from a single electrode in the ventral posteromedial thalamus. The results of simple paired stimulus experiments formed the basis for the development of a phenomenological model explicitly containing nonlinear elements observed experimentally. The phenomenological model was fit using datasets obtained with impulse train inputs, Poisson-distributed in time and uniformly varying in amplitude.
Main results: The phenomenological model explained 58% of the variance in the cortical response to out of sample patterns of thalamic microstimulation. Furthermore, while fit on trial-averaged data, the phenomenological model reproduced single trial response properties when simulated with noise added into the system during stimulus presentation. The simulations indicate that the single trial response properties were dependent on the relative sensitivity of the static nonlinearities in the two stages of the model, and ultimately suggest that electrical stimulation activates local circuitry through linear recruitment, but that this activity propagates in a highly nonlinear fashion to downstream targets.
Significance: The development of nonlinear dynamical models of neural circuitry will guide information delivery for sensory prosthesis applications, and more generally reveal properties of population coding within neural circuits.
Figures
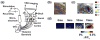





Similar articles
-
Thalamic state control of cortical paired-pulse dynamics.J Neurophysiol. 2017 Jan 1;117(1):163-177. doi: 10.1152/jn.00415.2016. Epub 2016 Oct 19. J Neurophysiol. 2017. PMID: 27760816 Free PMC article.
-
Voltage-sensitive dye imaging reveals improved topographic activation of cortex in response to manipulation of thalamic microstimulation parameters.J Neural Eng. 2012 Apr;9(2):026008. doi: 10.1088/1741-2560/9/2/026008. Epub 2012 Feb 13. J Neural Eng. 2012. PMID: 22327024 Free PMC article.
-
Electrical and Optical Activation of Mesoscale Neural Circuits with Implications for Coding.J Neurosci. 2015 Nov 25;35(47):15702-15. doi: 10.1523/JNEUROSCI.5045-14.2015. J Neurosci. 2015. PMID: 26609162 Free PMC article.
-
A new interpretation of thalamocortical circuitry.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1767-79. doi: 10.1098/rstb.2002.1164. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626011 Free PMC article. Review.
-
Thalamocortical processing of the head-direction sense.Prog Neurobiol. 2019 Dec;183:101693. doi: 10.1016/j.pneurobio.2019.101693. Epub 2019 Sep 21. Prog Neurobiol. 2019. PMID: 31550513 Review.
Cited by
-
Optogenetic feedback control of neural activity.Elife. 2015 Jul 3;4:e07192. doi: 10.7554/eLife.07192. Elife. 2015. PMID: 26140329 Free PMC article.
-
Multiscale low-dimensional motor cortical state dynamics predict naturalistic reach-and-grasp behavior.Nat Commun. 2021 Jan 27;12(1):607. doi: 10.1038/s41467-020-20197-x. Nat Commun. 2021. PMID: 33504797 Free PMC article.
-
Adaptive shaping of cortical response selectivity in the vibrissa pathway.J Neurophysiol. 2015 Jun 1;113(10):3850-65. doi: 10.1152/jn.00978.2014. Epub 2015 Mar 18. J Neurophysiol. 2015. PMID: 25787959 Free PMC article.
-
Sensory percepts induced by microwire array and DBS microstimulation in human sensory thalamus.Brain Stimul. 2018 Mar-Apr;11(2):416-422. doi: 10.1016/j.brs.2017.10.017. Epub 2017 Oct 27. Brain Stimul. 2018. PMID: 29126946 Free PMC article.
-
Combined Effects of Feedforward Inhibition and Excitation in Thalamocortical Circuit on the Transitions of Epileptic Seizures.Front Comput Neurosci. 2017 Jul 7;11:59. doi: 10.3389/fncom.2017.00059. eCollection 2017. Front Comput Neurosci. 2017. PMID: 28736520 Free PMC article.
References
-
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383(6603):815–819. - PubMed
-
- Bach-y-Rita P, Collins CC, Saunders FA, White B, Scadden L. Vision substitution by tactile image projection. Nature. 1969;221(5184):963–964. - PubMed
-
- Barros CG, Bittar RS, Danilov Y. Effects of electrotactile vestibular substitution on rehabilitation of patients with bilateral vestibular loss. Neurosci Lett. 2010;476(3):123–126. - PubMed
-
- Brugger D, Butovas S, Bogdan M, Schwarz C. Real-Time Adaptive Microstimulation Increases Reliability of Electrically Evoked Cortical Potentials. Biomedical Engineering, IEEE Transactions on. 2011;58(5):1483–1491. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
