A re-interpretation of the rate of tension redevelopment (k(TR)) in active muscle
- PMID: 24162314
- PMCID: PMC3909470
- DOI: 10.1007/s10974-013-9366-5
A re-interpretation of the rate of tension redevelopment (k(TR)) in active muscle
Abstract
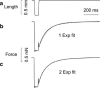
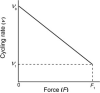
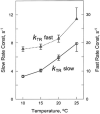
A slackening to zero tension by large length release (~20%) and a restretch of active muscle fibres cause a fall and a redevelopment in tension. According to the model of Brenner (Proc Natl Acad Sci USA 85(9):3265-3269, 1988), the rate constant of tension redevelopment (k TR) is the sum of attachment and detachment rate constants, hence is limited by the fast reaction. Here we propose a model in which, after restretch, cross-bridges cycle many times by stretching series elastic elements, hence k(TR) is limited by a slow reaction. To set up this model, we made an assumption that the stepping rate (v) decreases linearly with tension (F), which is consistent with the Fenn effect. The distance traveled by a cross-bridge stretches series elastic elements with stiffness σ. With these assumptions, we set up a first order differential equation, which results in an exponential time course with the rate constant k(TR) = ση(0)ν(0)(1 - λ)/F(1), where λ = ν(1)/ν(0), η = step size, the subscript 0 indicates unloaded condition, and the subscript 1 indicate isometric condition. We demonstrate that the ATP hydrolysis rate (=[myosin head]/ν(0)) is proportionate to k(TR) as the ambient temperature is changed, and that the published data fit to this relationship well if λ = 0.28. We conclude that k(TR) is limited by the cross-bridge turnover rate; hence it represents the rate constant of the slowest reaction of the cross-bridge cycle, i.e. the ADP isomerization step before ADP is released.
Figures



Similar articles
-
Effect of temperature on elementary steps of the cross-bridge cycle in rabbit soleus slow-twitch muscle fibres.J Physiol. 2001 Feb 15;531(Pt 1):219-34. doi: 10.1111/j.1469-7793.2001.0219j.x. J Physiol. 2001. PMID: 11179405 Free PMC article.
-
Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres.J Physiol. 2007 Mar 1;579(Pt 2):313-26. doi: 10.1113/jphysiol.2006.124164. Epub 2007 Jan 4. J Physiol. 2007. PMID: 17204497 Free PMC article.
-
The ATP hydrolysis and phosphate release steps control the time course of force development in rabbit skeletal muscle.J Physiol. 2005 Mar 15;563(Pt 3):671-87. doi: 10.1113/jphysiol.2004.078873. Epub 2004 Dec 20. J Physiol. 2005. PMID: 15611023 Free PMC article.
-
Induced potential model of muscular contraction mechanism and myosin molecular structure.Adv Biophys. 1999;36:107-58. doi: 10.1016/s0065-227x(99)80006-9. Adv Biophys. 1999. PMID: 10463074 Review.
-
What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres?J Muscle Res Cell Motil. 2003;24(2-3):127-38. doi: 10.1023/a:1026093212111. J Muscle Res Cell Motil. 2003. PMID: 14609024 Review.
Cited by
-
Omecamtiv mecarbil lowers the contractile deficit in a mouse model of nebulin-based nemaline myopathy.PLoS One. 2019 Nov 13;14(11):e0224467. doi: 10.1371/journal.pone.0224467. eCollection 2019. PLoS One. 2019. PMID: 31721788 Free PMC article.
-
Yank: the time derivative of force is an important biomechanical variable in sensorimotor systems.J Exp Biol. 2019 Sep 12;222(Pt 18):jeb180414. doi: 10.1242/jeb.180414. J Exp Biol. 2019. PMID: 31515280 Free PMC article.
-
The elementary step that generates force and sinusoidal analysis in striated muscle fibers.J Muscle Res Cell Motil. 2025 Jun;46(2):83-118. doi: 10.1007/s10974-025-09693-z. Epub 2025 Jul 7. J Muscle Res Cell Motil. 2025. PMID: 40622514 Free PMC article. Review.
-
Thick-Filament Extensibility in Intact Skeletal Muscle.Biophys J. 2018 Oct 16;115(8):1580-1588. doi: 10.1016/j.bpj.2018.08.038. Epub 2018 Sep 4. Biophys J. 2018. PMID: 30266320 Free PMC article.
-
Ex vivo Methods for Measuring Cardiac Muscle Mechanical Properties.Front Physiol. 2021 Jan 8;11:616996. doi: 10.3389/fphys.2020.616996. eCollection 2020. Front Physiol. 2021. PMID: 33488406 Free PMC article. Review.
References
-
- Brenner B. Muscle mechanics and biochemical kinetics. In: Squire JM, editor. Molecular mechanisms in muscular contraction. CRC; Boca Raton: 1990. pp. 77–149.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

