Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis
- PMID: 24162590
- PMCID: PMC4112447
- DOI: 10.1136/gutjnl-2012-304058
Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis
Abstract
Objective: Non-oxidative metabolism of ethanol (NOME) produces fatty acid ethyl esters (FAEEs) via carboxylester lipase (CEL) and other enzyme action implicated in mitochondrial injury and acute pancreatitis (AP). This study investigated the relative importance of oxidative and non-oxidative pathways in mitochondrial dysfunction, pancreatic damage and development of alcoholic AP, and whether deleterious effects of NOME are preventable.
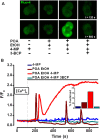
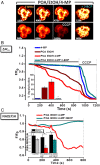
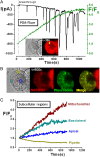
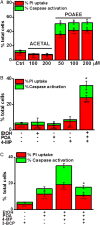
Design: Intracellular calcium ([Ca(2+)](C)), NAD(P)H, mitochondrial membrane potential and activation of apoptotic and necrotic cell death pathways were examined in isolated pancreatic acinar cells in response to ethanol and/or palmitoleic acid (POA) in the presence or absence of 4-methylpyrazole (4-MP) to inhibit oxidative metabolism. A novel in vivo model of alcoholic AP induced by intraperitoneal administration of ethanol and POA was developed to assess the effects of manipulating alcohol metabolism.
Results: Inhibition of OME with 4-MP converted predominantly transient [Ca(2+)](C) rises induced by low ethanol/POA combination to sustained elevations, with concurrent mitochondrial depolarisation, fall of NAD(P)H and cellular necrosis in vitro. All effects were prevented by 3-benzyl-6-chloro-2-pyrone (3-BCP), a CEL inhibitor. 3-BCP also significantly inhibited rises of pancreatic FAEE in vivo and ameliorated acute pancreatic damage and inflammation induced by administration of ethanol and POA to mice.
Conclusions: A combination of low ethanol and fatty acid that did not exert deleterious effects per se became toxic when oxidative metabolism was inhibited. The in vitro and in vivo damage was markedly inhibited by blockade of CEL, indicating the potential for development of specific therapy for treatment of alcoholic AP via inhibition of FAEE generation.
Keywords: ACUTE Pancreatitis; Alcohol-Induced Injury; Calcium; Ethanol; Pancreatic Damage.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
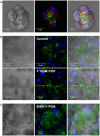

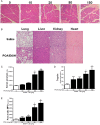
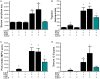
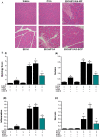
Similar articles
-
TRO40303 Ameliorates Alcohol-Induced Pancreatitis Through Reduction of Fatty Acid Ethyl Ester-Induced Mitochondrial Injury and Necrotic Cell Death.Pancreas. 2018 Jan;47(1):18-24. doi: 10.1097/MPA.0000000000000953. Pancreas. 2018. PMID: 29200128 Free PMC article.
-
Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release.Gut. 2017 Feb;66(2):301-313. doi: 10.1136/gutjnl-2015-309363. Epub 2015 Dec 7. Gut. 2017. PMID: 26642860 Free PMC article.
-
Docosahexaenoic acid inhibits ethanol/palmitoleic acid-induced necroptosis in AR42J cells.J Physiol Pharmacol. 2020 Jun;71(3). doi: 10.26402/jpp.2020.3.15. Epub 2020 Oct 15. J Physiol Pharmacol. 2020. PMID: 33077696
-
The role of fat and alcohol in acute pancreatitis: A dangerous liaison.Pancreatology. 2015 Jul;15(4 Suppl):S6-S12. doi: 10.1016/j.pan.2015.02.009. Epub 2015 Mar 28. Pancreatology. 2015. PMID: 25845855 Review.
-
Role of Ca2+ in pancreatic cell death induced by alcohol metabolites.J Gastroenterol Hepatol. 2006 Oct;21 Suppl 3:S14-7. doi: 10.1111/j.1440-1746.2006.04577.x. J Gastroenterol Hepatol. 2006. PMID: 16958662 Review.
Cited by
-
Risk factors for diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis.Front Med (Lausanne). 2024 Jan 9;10:1257222. doi: 10.3389/fmed.2023.1257222. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38264039 Free PMC article.
-
Recent Advances in Understanding the Complexity of Alcohol-Induced Pancreatic Dysfunction and Pancreatitis Development.Biomolecules. 2020 Apr 27;10(5):669. doi: 10.3390/biom10050669. Biomolecules. 2020. PMID: 32349207 Free PMC article. Review.
-
Uncovering the impact of alcohol on internal organs and reproductive health: Exploring TLR4/NF-kB and CYP2E1/ROS/Nrf2 pathways.Animal Model Exp Med. 2024 Aug;7(4):444-459. doi: 10.1002/ame2.12436. Epub 2024 Jun 9. Animal Model Exp Med. 2024. PMID: 38853347 Free PMC article. Review.
-
Early continuous blood purification affects TNF-α, IL-1β, and IL-6 in patients with severe acute pancreatitis via inhibiting TLR4 signaling pathway.Kaohsiung J Med Sci. 2022 May;38(5):479-485. doi: 10.1002/kjm2.12497. Epub 2022 Jan 20. Kaohsiung J Med Sci. 2022. PMID: 35049137 Free PMC article. Clinical Trial.
-
Different Effects of Alcohol on the Liver and the Pancreas.Function (Oxf). 2021 Feb 19;2(2):zqab008. doi: 10.1093/function/zqab008. eCollection 2021. Function (Oxf). 2021. PMID: 35330811 Free PMC article. No abstract available.
References
-
- Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology 2002;122:106–18 - PubMed
-
- Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 1986;231:497–9 - PubMed
-
- Werner J, Saghir M, Fernandez-del Castillo C, et al. Linkage of oxidative and nonoxidative ethanol metabolism in the pancreas and toxicity of nonoxidative ethanol metabolites for pancreatic acinar cells. Surgery 2001;129:736–44 - PubMed
-
- Laposata M. Fatty acid ethyl esters: ethanol metabolites which mediate ethanol-induced organ damage and serve as markers of ethanol intake. Prog Lipid Res 1998;37:307–16 - PubMed
-
- Best CA, Laposata M. Fatty acid ethyl esters: toxic non-oxidative metabolites of ethanol and markers of ethanol intake. Front Biosci 2003;8:e202–17 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
