Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential
- PMID: 24162662
- PMCID: PMC3890953
- DOI: 10.1038/cdd.2013.148
Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential
Abstract
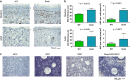
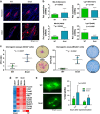
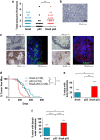
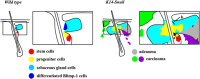
Expression of the EMT-inducing transcription factor Snail is enhanced in different human cancers. To investigate the in vivo role of Snail during progression of epithelial cancer, we used a mouse model with skin-specific overexpression of Snail. Snail transgenic mice spontaneously developed distinct histological subtypes of skin cancer, such as basal cell carcinoma, squamous cell carcinoma and sebaceous gland carcinoma. Development of sebaceous gland carcinomas strongly correlated with the direct and complete repression of Blimp-1, a central regulator of sebocyte homeostasis. Snail expression in keratinocyte stem cells significantly promotes their proliferation associated with an activated FoxM1 gene expression signature, resulting in a larger pool of Mts24-marked progenitor cells. Furthermore, primary keratinocytes expressing Snail showed increased survival and strong resistance to genotoxic stress. Snail expression in a skin-specific p53-null background resulted in accelerated formation of spontaneous tumours and enhanced metastasis. Our data demonstrate that in vivo expression of Snail results in de novo epithelial carcinogenesis by allowing enhanced survival, expansion of the cancer stem cell pool with accumulated DNA damage, a block in terminal differentiation and increased proliferation rates of tumour-initiating cells.
Figures


References
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev. 2003;3:362–374. - PubMed
-
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev. 2013;13:97–110. - PubMed
-
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
-
- De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 2005;65:6237–6244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

