Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa
- PMID: 24162806
- PMCID: PMC3934125
- DOI: 10.1262/jrd.2013-056
Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa
Abstract
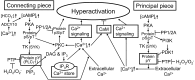
It is not until accomplishment of a variety of molecular changes during the transit through the female reproductive tract that mammalian spermatozoa are capable of exhibiting highly activated motility with asymmetric whiplash beating of the flagella (hyperactivation) and undergoing acrosomal exocytosis in the head (acrosome reaction). These molecular changes of the spermatozoa are collectively termed capacitation and promoted by bicarbonate, calcium and cholesterol acceptors. Such capacitation-promoting factors can stimulate intracellular cyclic AMP (cAMP) signal transduction in the spermatozoa. Meanwhile, hyperactivation and the acrosome reaction are essential to sperm fertilization with oocytes and are apparently triggered by a sufficient increase of intracellular Ca²⁺ in the sperm flagellum and head, respectively. Thus, it is necessary to investigate the relationship between cAMP signal transduction and calcium signaling cascades in the spermatozoa for the purpose of understanding the molecular basis of capacitation. In this review, I cover updated insights regarding intracellular cAMP signal transduction, the acrosome reaction and flagellar motility in mammalian spermatozoa and then account for possible roles of intracellular cAMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa.
Figures
Similar articles
-
Compartmentalization of distinct cAMP signaling pathways in mammalian sperm.J Biol Chem. 2013 Dec 6;288(49):35307-20. doi: 10.1074/jbc.M113.489476. Epub 2013 Oct 15. J Biol Chem. 2013. PMID: 24129574 Free PMC article.
-
Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm.Biol Reprod. 1999 Jul;61(1):76-84. doi: 10.1095/biolreprod61.1.76. Biol Reprod. 1999. PMID: 10377034
-
Role of signaling pathways in regulating the capacitation of mammalian spermatozoa.Cell Mol Biol (Noisy-le-grand). 2003 May;49(3):329-40. Cell Mol Biol (Noisy-le-grand). 2003. PMID: 12887085 Review.
-
Sperm Capacitation and Acrosome Reaction in Mammalian Sperm.Adv Anat Embryol Cell Biol. 2016;220:93-106. doi: 10.1007/978-3-319-30567-7_5. Adv Anat Embryol Cell Biol. 2016. PMID: 27194351 Review.
-
The CatSper channel modulates boar sperm motility during capacitation.Reprod Biol. 2017 Mar;17(1):69-78. doi: 10.1016/j.repbio.2017.01.001. Epub 2017 Jan 8. Reprod Biol. 2017. PMID: 28077244
Cited by
-
AMPK Function in Mammalian Spermatozoa.Int J Mol Sci. 2018 Oct 23;19(11):3293. doi: 10.3390/ijms19113293. Int J Mol Sci. 2018. PMID: 30360525 Free PMC article. Review.
-
Reconsideration of the evaluation criteria for bull ejaculated sperm motility in the context of rotation.J Reprod Dev. 2018 Oct 12;64(5):377-384. doi: 10.1262/jrd.2018-036. Epub 2018 Jun 27. J Reprod Dev. 2018. PMID: 29952339 Free PMC article.
-
Addition of seminal plasma proteins effecting the in vitro kinetic properties of canine spermatozoa.Vet Med (Praha). 2022 Apr 28;67(7):365-370. doi: 10.17221/73/2021-VETMED. eCollection 2022 Jul. Vet Med (Praha). 2022. PMID: 39100133 Free PMC article.
-
Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions.Int J Mol Sci. 2020 Apr 13;21(8):2701. doi: 10.3390/ijms21082701. Int J Mol Sci. 2020. PMID: 32295040 Free PMC article.
-
Central role of soluble adenylyl cyclase and cAMP in sperm physiology.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2610-20. doi: 10.1016/j.bbadis.2014.07.013. Epub 2014 Jul 24. Biochim Biophys Acta. 2014. PMID: 25066614 Free PMC article. Review.
References
-
- Bedford JM. Maturation, transport and fate of spermatozoa in the epididymis. In: Astwood EB, Greep RO (eds.), Handbook of Physiology, Section 7: Washington DC: American Physiological Society; 1975: 303–318.
-
- Orgebin-Crist MC, Danzo BJ, Davies J. Endocrine control of the development and maintenance of sperm fertilizing ability in the epididymis. In: Astwood EB, Greep RO (eds.), Handbook of Physiology, Section 7. Washington DC: American Physiological Society; 1975: 319–338.
-
- Hammerstedt RH, Parks JE. Changes in sperm surfaces associated with epididymal transit. J Reprod Fertil Suppl 1987; 34: 133–149 - PubMed
-
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (eds.), The Physiology of Reproduction. 2nd edition. New York: Raven Press; 1994: 189–317.
-
- Lewis B, Aitken RJ. Impact of epididymal maturation on the tyrosine phosphorylation patterns exhibited by rat spermatozoa. Biol Reprod 2001; 64: 1545–1556 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

