Wnt and planar cell polarity signaling in cystic renal disease
- PMID: 24162855
- PMCID: PMC4049898
- DOI: 10.4161/org.26766
Wnt and planar cell polarity signaling in cystic renal disease
Abstract
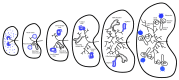
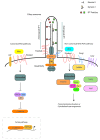
Cystic kidney diseases can cause end stage renal disease, affecting millions of individuals worldwide. They may arise early or later in life, are characterized by a spectrum of symptoms and can be caused by diverse genetic defects. The primary cilium, a microtubule-based organelle that can serve as a signaling antenna, has been demonstrated to have a significant role in ensuring correct kidney development and function. In the kidney, one of the signaling pathways that requires the cilium for normal development is Wnt signaling. In this review, the roles of primary cilia in relation to canonical and non-canonical Wnt/PCP signaling in cystic renal disease are described. The evidence of the associations between cilia, Wnt signaling and cystic renal disease is discussed and the significance of planar cell polarity-related mechanisms in cystic kidney disease is presented. Although defective Wnt signaling is not the only cause of renal disease, research is increasingly highlighting its importance, encouraging the development of Wnt-associated diagnostic and prognostic tools for cystic renal disease.
Keywords: Wnt signalling; cilia; cystic renal disease; kidney morphogenesis; planar cell polarity.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
