Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study
- PMID: 24162898
- PMCID: PMC3972021
- DOI: 10.1161/CIRCHEARTFAILURE.113.000568
Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study
Abstract
Background: Atrial fibrillation (AF) is common among patients with heart failure and preserved ejection fraction (HFpEF), but its clinical profile and impact on exercise capacity remain unclear. RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in HFpEF) was a multicenter randomized trial testing the impact of sildenafil on peak VO2 in stable outpatients with chronic HFpEF. We sought to compare clinical features and exercise capacity among patients with HFpEF who were in sinus rhythm (SR) or AF.
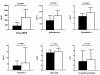
Methods and results: RELAX enrolled 216 patients with HFpEF, of whom 79 (37%) were in AF, 124 (57%) in SR, and 13 in other rhythms. Participants underwent baseline cardiopulmonary exercise testing, echocardiogram, biomarker assessment, and rhythm status assessment before randomization. Patients with AF were older than those in SR but had similar symptom severity, comorbidities, and renal function. β-blocker use and chronotropic indices were also similar. Despite comparable left ventricular size and mass, AF was associated with worse systolic (lower EF, stroke volume, and cardiac index) and diastolic (shorter deceleration time and larger left atria) function compared with SR. Pulmonary artery systolic pressure was higher in AF. Patients with AF had higher N-terminal pro-B-type natriuretic peptide, aldosterone, endothelin-1, troponin I, and C-telopeptide for type I collagen levels, suggesting more severe neurohumoral activation, myocyte necrosis, and fibrosis. Peak VO2 was lower in AF, even after adjustment for age, sex, and chronotropic response, and VE/VCO2 was higher.
Conclusions: AF identifies an HFpEF cohort with more advanced disease and significantly reduced exercise capacity. These data suggest that evaluation of the impact of different rate or rhythm control strategies on exercise tolerance in patients with HFpEF and AF is warranted.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00763867.
Keywords: atrial fibrillation; exercise; heart failure.
Figures

References
-
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. - PubMed
-
- Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA, Investigators C. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the candesartan in heart failure-assessment of reduction in mortality and morbidity (charm) program. Journal of the American College of Cardiology. 2006;47:1997–2004. - PubMed
-
- Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, Cleland JG. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (cafe-ii study) Heart. 2009;95:924–930. - PubMed
-
- Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL084891/HL/NHLBI NIH HHS/United States
- U01 HL084931/HL/NHLBI NIH HHS/United States
- U01HL084889/HL/NHLBI NIH HHS/United States
- U01HL084861/HL/NHLBI NIH HHS/United States
- U01 HL084875/HL/NHLBI NIH HHS/United States
- T32 HL007111/HL/NHLBI NIH HHS/United States
- U01HL084931/HL/NHLBI NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- U10 HL110262/HL/NHLBI NIH HHS/United States
- U01 HL084899/HL/NHLBI NIH HHS/United States
- U10 HL110337/HL/NHLBI NIH HHS/United States
- U01HL084891/HL/NHLBI NIH HHS/United States
- U01HL084875/HL/NHLBI NIH HHS/United States
- U01 HL084890/HL/NHLBI NIH HHS/United States
- UL1TR000135/TR/NCATS NIH HHS/United States
- U54 MD007588/MD/NIMHD NIH HHS/United States
- UL1TR000454/TR/NCATS NIH HHS/United States
- U10HL110262/HL/NHLBI NIH HHS/United States
- U01HL084890/HL/NHLBI NIH HHS/United States
- U01 HL084861/HL/NHLBI NIH HHS/United States
- U10 HL084904/HL/NHLBI NIH HHS/United States
- R01 HL105418/HL/NHLBI NIH HHS/United States
- U01HL084907/HL/NHLBI NIH HHS/United States
- U01HL084877/HL/NHLBI NIH HHS/United States
- U01 HL084907/HL/NHLBI NIH HHS/United States
- U01 HL084904/HL/NHLBI NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01HL084904/HL/NHLBI NIH HHS/United States
- U01 HL084877/HL/NHLBI NIH HHS/United States
- U01HL084899/HL/NHLBI NIH HHS/United States
- U01 HL084889/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

