Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature
- PMID: 24163065
- PMCID: PMC4090244
- DOI: 10.1161/CIRCULATIONAHA.113.003000
Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature
Abstract
Background: The generation of vascular progenitors (VPs) from human induced pluripotent stem cells (hiPSCs) has great potential for treating vascular disorders such as ischemic retinopathies. However, long-term in vivo engraftment of hiPSC-derived VPs into the retina has not yet been reported. This goal may be limited by the low differentiation yield, greater senescence, and poor proliferation of hiPSC-derived vascular cells. To evaluate the potential of hiPSCs for treating ischemic retinopathies, we generated VPs from a repertoire of viral-integrated and nonintegrated fibroblast and cord blood (CB)-derived hiPSC lines and tested their capacity for homing and engrafting into murine retina in an ischemia-reperfusion model.
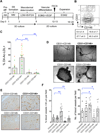
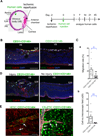
Methods and results: VPs from human embryonic stem cells and hiPSCs were generated with an optimized vascular differentiation system. Fluorescence-activated cell sorting purification of human embryoid body cells differentially expressing endothelial/pericytic markers identified a CD31(+)CD146(+) VP population with high vascular potency. Episomal CB-induced pluripotent stem cells (iPSCs) generated these VPs with higher efficiencies than fibroblast-iPSC. Moreover, in contrast to fibroblast-iPSC-VPs, CB-iPSC-VPs maintained expression signatures more comparable to human embryonic stem cell VPs, expressed higher levels of immature vascular markers, demonstrated less culture senescence and sensitivity to DNA damage, and possessed fewer transmitted reprogramming errors. Luciferase transgene-marked VPs from human embryonic stem cells, CB-iPSCs, and fibroblast-iPSCs were injected systemically or directly into the vitreous of retinal ischemia-reperfusion-injured adult nonobese diabetic-severe combined immunodeficient mice. Only human embryonic stem cell- and CB-iPSC-derived VPs reliably homed and engrafted into injured retinal capillaries, with incorporation into damaged vessels for up to 45 days.
Conclusions: VPs generated from CB-iPSCs possessed augmented capacity to home, integrate into, and repair damaged retinal vasculature.
Keywords: blood supply; diabetic retinopathy; embryonic stem cells; induced pluripotent stem cells; reperfusion injury; stem cells; transplantation.
Conflict of interest statement
Figures





References
-
- Paxhia MJ, Ting TD, Fekrat S. Ischemic central retinal vein occlusion and retinitis pigmentosa: Lower risk of neovascularization? Retina. 2001;21:179–180. - PubMed
-
- Awdeh RM, Elsing SH, Deramo VA, Stinnett S, Lee PP, Fekrat S. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item national eye institute visual function questionnaire. Br J Ophthalmol. 2010;94:319–323. - PubMed
-
- Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116:589–597. - PubMed
-
- Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: Similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. - PubMed
-
- Kim SY, Johnson MA, McLeod DS, Alexander T, Otsuji T, Steidl SM, Hansen BC, Lutty GA. Retinopathy in monkeys with spontaneous type 2 diabetes. Invest Ophthalmol Vis Sci. 2004;45:4543–4553. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HL100397/HL/NHLBI NIH HHS/United States
- R01-EY09357/EY/NEI NIH HHS/United States
- T32 CA060441/CA/NCI NIH HHS/United States
- CA60441/CA/NCI NIH HHS/United States
- U01 HL099775/HL/NHLBI NIH HHS/United States
- R01 EY009357/EY/NEI NIH HHS/United States
- R03 HL096220/HL/NHLBI NIH HHS/United States
- U01HL099775/HL/NHLBI NIH HHS/United States
- U01HL100397/HL/NHLBI NIH HHS/United States
- R01 CA043318/CA/NCI NIH HHS/United States
- K08 HL077595/HL/NHLBI NIH HHS/United States
- EY01761/EY/NEI NIH HHS/United States
- R01 EY023962/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

